Introduction
As an ailment, osteoarthrosis is an important social and biological issue. According to the data from the published sources worldwide, about 10% of the world population suffers from osteoarthrosis (OA) [1-3]. Federal Statistics Service reported OA among the leading causes of disability in Russia [4], with a steadily increasing morbidity trend over the past two decades, virtually doubling the number of such patients over time [5, 6]. Epidemiological survey data stated that 13% of Russian Federation population has already been ill with OA of the knee and/or hip joint [7, 8].
Inflammation is considered traditionally the cause of OA [9, 10], while activation of free-radical oxidation processes initiates and promotes degenerative changes in the hyaline cartilage. As a result, metabolic impairment of the connective tissue takes place [10, 11]. The latter, in turn, results in articular cartilage degeneration, lipping, inflammation, synovial compaction, as well as damaged ligaments and articular capsule, all of which lead to chronic pain, stiffness, deformation, and movement disorders [12].
There are multiple treatment approaches to OA. Previously, administering cartilage protectors, glucocorticoids, and non-steroidal anti-inflammatory agents was considered to be the most efficient tactic [13, 14]. However, these medication groups have a number of disadvantages, as well as some adverse side effects [15].
The intra-articular injections of autologous platelet-rich plasma (PRP) were chosen in the process of searching for novel methods of pathogenetic OA therapy [16-19]. The aforementioned methodology had been first applied in 1965 at California University of Science and Medicine to stimulate osteogenesis in patients with maxillary injury [20].
PRP is a highly active agent containing, among others, high concentrations of the platelet-derived growth factor (PDGF), platelet insulin-like growth factor (IGF), vascular endothelial growth factor (VEGF) and transforming growth factor beta (TGF-β). These substances, acting locally on pathological lesion, induce an intensive anti-inflammatory effect stimulating tissue regeneration [23-25]. It was also proved that there are substances with both chondrogenic and osteogenic properties in the platelet alpha-granules, which are also present in PRP [21, 22].
Considering the discussed prerequisites, the urgency of searching for novel highly effective and safe methods of osteoarthritis treatment, and significant therapeutic potential of using autologous platelet-rich plasma, the goal of this clinical study was to evaluate the effectiveness of autologous platelet-rich plasma injections in osteoarthrosis patient treatment.
Material and Methods
Study design
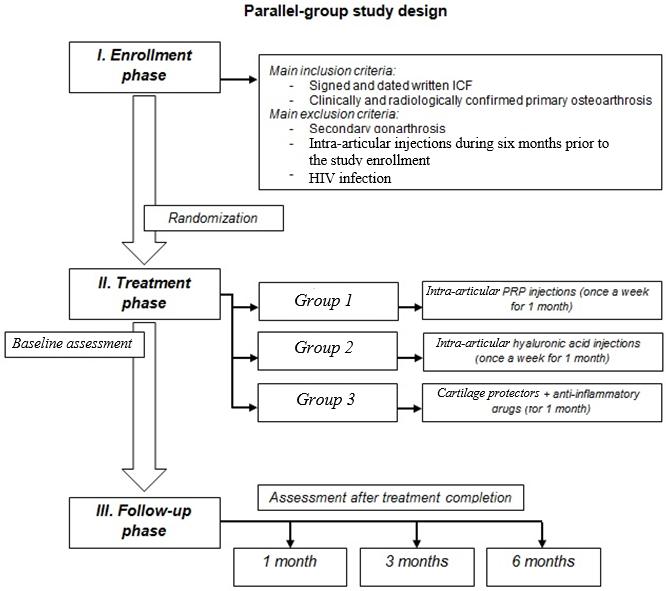
This open-label parallel-group study was a pilot randomized controlled trial aimed to evaluate the effectiveness of autologous PRP injections in OA patient treatment. The study design was based on the analysis of the treatment outcomes for 128 patients with knee joint arthrosis. The clinical diagnosis was identified sensu the Congress of Russian Rheumatologists (1993) [26]. Our research design is presented schematically in the Figure 1. The study inclusion and exclusion criteria were as follows:
Inclusion criteria:
- Signed and dated written informed consent form (WICF) for the study;
- Clinically and radiologically confirmed primary osteoarthritis of the knee joint – grade 3, according to Kellgren and Lawrence classification criteria [29].
Exclusion criteria:
- Secondary gonarthrosis;
- Intra-articular pharmaceutical drug injections during six months prior to the study enrollment;
- HIV infection;
- Patient’s disagreement to participate in the study.
Figure 1. Study design.
All eligible patients were randomly allocated to three groups of comparable baseline traits: age and gender composition, illness duration, and severity of functional and morphological disorders.
Group 1 (experimental treatment) included 47 patients. Treatment plan in this group included intra-articular PRP injections once a week for 1 month (i.e., a total of 4 PRP injections 7 days apart). We used the conventional preparation technique for autologous plasma with platelet concentration approximately 800×109 per liter [27].
Group 2 (active control) comprised of 38 study subjects. The provided treatment included hyaluronic acid injections into the joint cavity once a week for 1 month.
Study assessments
The effectiveness of treatment was assessed from clinical and laboratory data. Among all other clinical parameters, pain intensity in the joint (at rest or while walking) was rated using the Visual Analog Scale (VAS). We used standard VAS, presented as a 10 cm line with descriptive anchors along it (0 = no pain; 1-3 = mild pain; 4-6 = moderate to severe pain; 7-9 = very severe pain; 10 = worst possible pain). Functional state of affected joints was scored sensu Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and Lequesne algofunctional index [30, 31]. Patient monitoring was carried out at the beginning of the treatment period (baseline value), as well as 1, 3 and 6 months after the completion of the treatment course.
The laboratory data included assessment of the dynamic pattern for superoxide dismutase (SOD) content and erythrocyte catalase activity. Similar to clinical evaluation, laboratory tests were performed at the beginning of the treatment period (baseline value), as well as 1, 3 and 6 months after the treatment completion.
To evaluate and compare the effectiveness of intra-articular PRP injections versus standard treatment, the statistical analyses of collected data were performed, using Statistica software, version 10.0 (StatSoft, USA). Prior to statistical comparisons, we tested our data for normality – both graphically and via Shapiro–Wilk test. The descriptive statistics were presented as mean values (M), standard deviations and standard errors (m). To compare the parameters between the study groups, the ANOVA test was conducted. The patterns were considered statistically significant at p-values≤0.05.
Results
The dynamics of parameters characterizing the clinical effectiveness is presented in Tables 1-3.
Table 1. Pain intensity dynamics sensu VAS in OA patients
|
Patient groups |
Pain scale |
||||
|
Baseline values |
1 month |
3 months |
6 months |
||
|
At rest |
Group 1 (n=47) |
5.8±0.3 |
4.6±0.4 |
3.2±0.1*,**,*** |
2.1±0.2*,**,*** |
|
Group 2 (n=38) |
5.4±0.5 |
4.1±0.3* |
3.8±0.2* |
3.3±0.3* |
|
|
Group 3 (n=43) |
5.7±0.3 |
5.0±0.4 |
4.3±0.3 |
3.7±0.2* |
|
|
During walking |
Group 1 (n=47) |
6.8±0.1 |
5.8±0.3 |
4.5±0.1*,**,*** |
4.3±0.2*,** |
|
Group 2 (n=38) |
6.7±0.3 |
5.4±0.3 |
5.0±0.2* |
4.8±0.1* |
|
|
Group 3 (n=43) |
6.8±0.2 |
6.1±0.4 |
5.1±0.2* |
4.2±0.1* |
|
* – p≤0.05 compared with the appropriate baseline value; ** – p≤0.05 compared with Group 2 within the same follow-up period; *** – p≤0.05 compared with Group 3 within the same follow-up period.
Table 2. WOMAC dynamics in OA patients
|
Patient groups |
Symptoms |
Baseline values |
1 month |
3 months |
6 months |
|
Group 1 (n=47) |
Pain severity |
161.2±34.1 |
105.2±24.1 |
61.17±19.5* |
39.24±15.1* |
|
Motion stiffness |
63.2±25.1 |
30.7±19.1 |
18.5±14.3* |
11.4±12.1* |
|
|
Functional impairment |
224.7±36.2 |
149.4±23.2 |
103.4±18.9* |
55.4±14.1* |
|
|
Group 2 (n=38) |
Pain severity |
158.8±38.5 |
88.8±18.2* |
75.7±17.1* |
42.5±11.9* |
|
Motion stiffness |
65.7±24.7 |
40.4±19.9 |
24±14.1 |
15.8±14.5* |
|
|
Functional impairment |
226.8±40.1 |
151±26.1 |
121.1±15.6* |
60.4±16.8* |
|
|
Group 3 (n=43) |
Pain severity |
155.±39.1 |
121.5±21.4 |
88.4±15.1 |
55.4±11.4* |
|
Motion stiffness |
66.7±28.1 |
49.1±20.4 |
32.8±16.4 |
19.8±16.1* |
|
|
Functional impairment |
226.8±31.9 |
168.1±19.3 |
95.3±18.1* |
71.2±15.1* |
* – p≤0.05 compared with the appropriate baseline value; ** – p≤0.05 compared with Group 2 within the same follow-up period; *** – p≤0.05 compared with Group 3 within the same follow-up period.
Table 3. Lequesne index dynamics in OA patients
|
Patient groups |
Baseline values |
1 month |
3 months |
6 months |
|
Group 1 (n=47) |
12.1±0.3 |
8.1±0.2*,**,*** |
6.4±0.3*,*** |
4.1±0.6*,*** |
|
Group 2 (n=38) |
11.9±0.5 |
9.4±0.3* |
6.8±0.2* |
5.5±0.1* |
|
Group 3 (n=43) |
11.9±0.2 |
10.1±0.3 |
9.5±0.3* |
6.8±0.4* |
* – p≤0.05 compared with the appropriate baseline value; ** – p≤0.05 compared with Group 2 within the same follow-up period; *** – p≤0.05 compared with Group 3 within the same follow-up period.
In the course of our study, we demonstrated that the differences in pain intensity among the patients from the study groups 1, 2 and 3 were already quite obvious after just a month in the post-treatment period, even though the differences from the baseline values were not statistically significant on most occasions (Table 1). Patients in Group 2 exhibited the best response to the treatment after 1 month: their pain intensities, according to VAS, were 4.1±0.3 (p=0.02) at rest and 5.4±0.3 (p=0.003) during walking. Reduction in pain intensity was observed in this group, compared both with the baseline value within this group and with other treatment groups. However, 3 months after the treatment completion, the situation has changed. The lowest pain intensities sensu VAS were registered in Group 1 with PRP administering: 3.2±0.1 (p<0.001) at rest and 4.5±0.1 (p<0.001) during walking. These results were significantly lower, compared with other groups and the baseline values of this group. Such dynamics remained in place even 6 months after the treatment course completion. The analysis of collected data suggested that administering hyaluronic acid has inhibited the pain syndrome in the fastest way. However, long-term results demonstrated the superiority of PRP injections for pain relief.
The analysis of WOMAC values showed similar results (Table 2), although no statistically significant differences were found between the study groups. A month after treatment, the most effective technique of pain relief involved intra-articular injections of hyaluronic acid. In Group 2, the pain intensity sensu WOMAC was 88.8±18.2, which was lower than in other treatment groups at the same period. However, as for joint stiffness and functional impairment, patients in Group 1 had the best outcomes: 30.7±19.1 and 149.4±23.2, respectively. In this case, similar tendency was observed throughout the whole follow-up period of the study. Also, pain intensity after 3 months in the group with PRP administering (61.71±19.5) was lower than in other groups, and compared with the previous assessment points within the group. These findings may suggest that PRP injections effectively relieved the pain, as well as reduced motion stiffness and functional lesions in OA patients. Thus, WOMAC values have demonstrated the superiority, or at least noninferiority, of PRP administering, compared with conventional treatment via anti-inflammatory medications and hyaluronic acid.
The dynamics of disability changes exhibited the following trend (Table 3): in a month after therapy completion, for patients from all study groups, the Lequesne index showed the significant degree of disability and varied from 8.1±0.2 to 10.1±0.3. However, the smallest value of the disability index was observed in Group 1 (8.1±0.2), which was significantly lower than in other study groups at the same period of time (p<0.001, compared with both Group 2 and Group 3). Three months after the treatment completion, Lequesne index values in patients of Groups 1 and 2 have decreased moderately (6.4±0.3 and 6.8±0.2, respectively), while in Group 3, receiving standard treatment, its value was still high (9.5±0.3). After 6 post-treatment months, the following outcomes were obtained: the patients in Group 1 had the minimum Lequesne index value of 4.1±0.6 (p<0.001, compared with Group 3), which matched the mild degree of joint dysfunction; subjects in Group 2 had its mean value of 5.5±0.6 (also considered mild dysfunction; and in Group 3, the index value was 6.8±0.4, implying a moderate to severe degree of disability. Based on provided data, it could be noted that PRP injections have significantly improved Lequesne index scores in OA patients, whereas the patients with standard treatment scheme (Group 3) exhibited the poorest improvement of disability. Based on this finding, we propose that PRP injections improve the quality of life in OA patients. However, further studies are needed to assess the PRP effect on long-term quality of life.
One of the complex pathogenesis elements of cartilaginous degenerative changes in OA is an activation of the free-radical oxidation and lipid peroxidation [32]. Hence, reliable indicators reflecting the inflammatory response intensity in OA are superoxide dismutase (SOD) content and erythrocyte catalase activity level (Table 4). The baseline concentrations of SOD and scavenger enzymes in all study groups were far from their normal ranges (1092−1817 U/g Hb and 18.4−25.0 mU/g Hb, respectively [33]), which confirmed the ailment duration and severity of inflammatory process. In a month after the treatment course, an increase in SOD content of 877.2±124.2 was observed in patients of Group 1, compared both with the baseline value and with the parameter values in Groups 2 and 3 during the same period (760.2±115.3 and 770.2±104.2, respectively), although these changes were not statistically significant. The same dynamics was observed during the further follow-up period. In 3 months after the treatment completion, SOD content was normalized in Group 1 (1241.1±115.7, p<0.001, compared with both Group 2 and Group 3), while in Groups 2 and 3, this parameter values were still far from their normal range (807.8±107.1 and 801.1±105.2, correspondingly).
Table 4. Dynamics of biochemical parameters in OA patients
|
Patient groups |
Parameters |
Baseline values |
1 month |
3 months |
6 months |
|
Group 1 (n=47) |
Superoxide dismutase |
757.8±194.1 |
877.2±124.2 |
1241.1±115.7*,**,*** |
1378.4±175.1*,**,*** |
|
Erythrocyte catalase |
54.9±11.1 |
35.2±13.4 |
27.4±9.8* |
22.4±8.1* |
|
|
Group 2 (n=38) |
Superoxide dismutase |
704.1±207.1 |
760.2±115.3 |
807.8±107.1 |
850.4±112.8 |
|
Erythrocyte catalase |
58.1±12.8 |
50.7±11.5 |
40.4±5.1 |
35.4±3.1* |
|
|
Group 3 (n=43) |
Superoxide dismutase |
728.4±197.4 |
770.2±104.2 |
801.1±105.2 |
842.7±114.3 |
|
Erythrocyte catalase |
56.2±13.4 |
51.5±14.3 |
42.1±6.8 |
36.8±3.8 |
* – p≤0.05 compared with the appropriate baseline value; ** – p≤0.05 compared with Group 2 within the same follow-up period; *** – p≤0.05 compared with Group 3 within the same follow-up period.
Similar trend was observed during the assessment of changes in erythrocyte catalase activity level. The reduction of scavenger enzyme content to 35.2±13.4 in patients of Group 1, albeit not statistically significant, was noted already in a month after the treatment completion. However, this trend continued, and already in 3 months, the parameter has reached its normal values; whereas in Groups 2 and 3, decrease of catalase activity level was less prominent. A month after treatment, it was 50.7±11.5 and 51.5±14.3 in Group 2 and Group 3, respectively, which did not differ significantly from the baseline values. Over the entire post-treatment follow-up study period, we did not observe this parameter approaching normal values. Analysis of the laboratory data suggested that intra-articular PRP injections had the most apparent anti-inflammatory effect in OA patients, while the hyaluronic acid and chondroitin sulfate did not affect the dynamics of the inflammatory process noticeably.
Discussion
Choosing an effective management and treatment plan for OA is still controversial and debatable. Quite often, in this disease, administering hyaluronic acid injections is preferred, due to relatively rapid pain relief. However, habitually, the remission period lasts no more than 6−8 months, after which the second therapy course is required [13, 34]. The available published data indicated a large number of side effects caused by hyaluronic acid injections, both local and systemic, which can be reasonable explained by an abundant blood supply and lymph flow in the area of the synovial membrane [35]. Among oral medications, a group of cartilage protectors stands out. Their mechanism of action is aimed directly at the regeneration of cartilage tissue. However, this technique has several disadvantages, such as limited bioavailability of cartilage protectors, their low effectiveness in terms of pain relief, and a long period from the start of their administering to some substantial clinical effect [36, 37].
Based on the outcomes of this pilot study, we could recommend to administer the autologous human PRP as a treatment for OA. During the study, we discovered that PRP injections had a more evident effect, compared with hyaluronic acid and a standard therapy plan that included cartilage protectors for pain relief. Already in a month, the pain intensity in Group 1, according to the VAS score, was significantly lower than Groups 2 and 3, both at rest and during walking. A similar pattern was observed when evaluating the WOMAC and Lequesne index values.
We established a similar trend when analyzing biochemical changes. PRP exhibited superiority and greater effectiveness, probably, due to its mechanism of action. It is obvious that autologous PRP blocks the inflammatory process in the joints, thereby inhibiting the development of oxidative stress, which is the most pathogenetically substantiated treatment for OA. On the other hand, the action of hyaluronic acid is directed at the synovial fluid, blocking the inflammatory mediators and stimulating the cartilage synthesis. In turn, cartilage protectors usually affect the destruction process of macromolecular structures, while stimulating recovery in interstitial tissue and articular cartilage tissue, along with normalization of hyaluronic acid biosynthesis.
To date, numerous studies described the positive effect of the autologous plasma use in the treatment of degenerative disorders of the musculoskeletal system. However, the mechanism of PRP action has not yet been fully understood. Sampson S. et al. has proved that autologous plasma, injected into the ears of rabbits as an injectable scaffold, stimulated chondrogenesis. These results were confirmed by the data of the biopsy histological examination and magnetic resonance imaging [38, 39]. According to Anitua E. et al., use of PRP in patients with osteoarthritis of the knee joints resulted in increase of the hyaluronic acid content and angiogenesis [40].
The results of the study by Chahla J. et al. demonstrated a statistically significant superiority of PRP, compared with hyaluronic acid injections, in the treatment of OA patients. The use of autologous plasma made it possible to achieve a more prolonged effect: after six months, the pain intensity was significantly lower than in patients treated with hyaluronic acid [41]. There is also reliable data on the successful use of PRP in lateral epicondylitis. The obtained results indicated a significant improvement of the joint function in patients treated with autologous plasma [42].
Therefore, the results of many studies implied the effectiveness of the autologous plasma use in the treatment of inflammatory and degenerative diseases of the musculoskeletal system. However, the mechanism of PRP action has not yet been fully understood, and possible adverse side effects and contraindications have not been yet studied. Hence, PRP administering in OA patients is a promising research area that requires further careful analysis.
Conclusion
The use of autologous PRP in the treatment of OA patients allowed us obtaining the superior, or at least noninferior, outcome according to both clinical (relieving pain syndrome, and reducing both functional impairment and motion stiffness) and laboratory (increasing SOD content and decreasing erythrocyte catalase activity level) parameters, compared with active comparator treatment schemes. Based on the results of this pilot study, with the certain limitations, PRP therapy could be considered an optimal method for treating OA patients.
Limitations
Although this clinical pilot study has demonstrated the effectiveness of intra-articular PRP injections for patients with osteoarthritis on a fairly large sample of patients, the obtained results should be interpreted with some limitations. In particular, the presented study was not a blinded and sufficiently controlled trial. Also, the goal of this pilot study did not involve a comprehensive assessment of the safety and tolerability of various treatment schemes, as well as evaluation of the impact on the quality of life in patients. These and other parameters require more detailed studies. Finally, for some of studied parameters, only hypothetical trends rather than confirmed statistically significant differences were shown. Partially, this was due to a fairly large variability of the studied parameters. As a consequence, a further study, completed on a much larger sample of patients, as well as their additional standardization by the baseline values of parameters, would likely lead to an increase in the statistical power and greater reliability of prospective results.
Ethical approval
All patients, willing to be included in clinical study, provided written informed consent prior to the study and any study-related procedures. All procedures involving human participants, performed during the study, were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Declaration of Helsinki and its later amendments. Study protocol and WICF for study participants were approved by the Institutional Review Board (IRB) (No. 6 of June 4, 2020).
Acknowledgments
This study was partly supported by the V.I. Vernadsky Crimean Federal University Development Program for 2015-2024 (Research and Technological Development Topic No. VG07/2020).
Conflict of interest
The authors declare no conflicts of interest pertaining to this project.
Group 3 (active control) encompassed 43 patients receiving conventional treatment: cartilage protectors (chondroitin sulfate [Alflutop ®] was administered via 1 mL intramuscular injections once a day for 20 days), non-steroidal anti-inflammatory agents (Meloxicam 15 mg in intramuscular injections twice a day for 5 days, followed by Celecoxib 200 mg orally twice daily for 14 days), proton-pump inhibitors (Omeprazole 20 mg twice a day orally for the entire period of the non-steroidal anti-inflammatory agents dosing), and physical therapy [28].
- Zolotovskaya IA, Davydkin IL. Vitamin D – prognostic marker of the risk of exacerbation in patients older than 60 years with osteoarthritis of the knee (results of the observation program DIANA). Ter Arkh 2019; 91(5): 103-110.Russian. https://doi.org/10.26442/00403660.2019.05.000237.
- Razumov AN, Puriga AO, Yurova OV. The results of the combined application of extracorporeal shock-wave therapy and radon baths during the rehabilitative treatment of the patients presenting with gonarthrosis. Problems of Balneology, Physiotherapy and Exercise Therapy 2015; 92(5): 35-39 https://doi.org/10.17116/kurort2015535-39.
- Burkard T, Rauch M, Spoendlin J, Prieto-Alhambra D, Jick SS, Meier CR et al. Risk of hand osteoarthritis in new users of hormone replacement therapy: A nested case-control analysis. Maturitas 2020; 132:17-23. https://doi.org/10.1016/j.maturitas.2019.11.006.
- Pérez-García S, Carrión M, Gutiérrez-Cañas I, Villanueva-Romero R, Castro D, Martínez C. et al. Profile of Matrix-Remodeling Proteinases in Osteoarthritis: Impact of Fibronectin. Cells. 2020; 9(1): 40. https://doi.org/10.3390/cells9010040.
- Ewart D, Harper L, Gravely A, Miller RA, Carlson CS, Loeser RF. Naturally occurring osteoarthritis in male mice with an extended lifespan. Connect Tissue Res. 2020; 61(1): 95-103. https://doi.org/10.1080/03008207.2019.1635590.
- Alekseeva LI, Taskina EA, Kashevarova NG. Osteoarthritis: epidemiology, classification, risk factors, and progression, clinical presentation, diagnosis, and treatment. Modern Rheumatology Journal 2019; 13(2): 9-21. Russian. https://doi.org/10.14412/1996-7012-2019-2-9-21.
- Kashevarova NG, Alekseyeva LI, Anikin SG, Demin NV, Smirnov AV, Nasonov EL. Knee joint osteoarthritis: risk factors for disease progression during 5-year prospective observation. Issues of organization and informatization health care 2016; (5): 144-149. Russian. https://elibrary.ru/item.asp?id=29855353.
- Golovach IY, Yehudina YD. Osteoarthritis and gout: evidence of relationship and possible therapeutic interventions. Trauma 2019; 20(3): 5-16. Russian. https://doi.org/10.22141/1608-1706.3.20.2019.172088.
- Vaskova NV, Lesnyak OM. Hyaluronic acid in treatment of knee osteorthritis. Russian Family Doctor 2014; 18(3): 29-34. Russian. https://doi.org/10.17816/RFD2014329-34.
- Elovikova ES, Bugrova OV, Leyzerman VG, Krasikov SI. Dynamics of antioxidative protection in patients with osteoarthrosis treated by glucosamine and chondroitin. Kazan Medical Journal 2009; 90(6): 784-788. Russian. https://elibrary.ru/item.asp?id=12979838.
- Kim C, Keating A. Cell Therapy for Knee Osteoarthritis: Mesenchymal Stromal Cells. Gerontology 2019; 65(3): 294-298. https://doi.org/10.1159/000496605.
- Badokin VV. The place and value of nonsteroidal anti-inflammatory drugs in the therapy of osteoarthritis. Sovremennaya Revmatologiya 2016; 10(1): 67-71. Russian. https://doi.org/10.14412/1996-7012-2016-1-67-72.
- Helo MJ, Akhtiamov IF, Abdullah AM, Said FM. Treatment of arthrosis of the knee, current trends and issues. Practical Medicine 2018; (7-1): 48–53. Russian. https://elibrary.ru/item.asp?id=36323465.
- Sarmanova A, Hall M, Moses J, Doherty M, Zhang W. Synovial changes detected by ultrasound in people with knee osteoarthritis – a meta-analysis of observational studies. Osteoarthritis and Cartilage 2016; 24(8): 1376-1383. Russian. https://doi.org/10.1016/j.joca.2016.03.004.
- Mironova AS, Klenin AA. PRP-therapy in treatment of osteoarthrosis of the branch joint. In: Collection of Abstracts of the V All-Russian Conference of Young Scientists and students with international participation "VOLGAMEDSCIENCE": materials conferences. Nizhny Novgorod, Russia: PIMU Publishing House, 2019: 89-90. Russian. https://elibrary.ru/item.asp?id=37306463.
- Llerena-Fernández EL, Ortiz-Culca FA. Métodos de diagnóstico y tratamiento actuales de la osteoartritis de la articulación temporomandibular: una revisión de la literatura. Rev Cient Odontol (Lima) 2019; 7(1): 121-131. Spanish. https://doi.org/10.21142/2523-2754-0701-2019-121-131.
- Glynn LG, Mustafa A, Casey M, Krawczyk J, Blom J, Galvin R. et al. Platelet-rich plasma (PRP) therapy for knee arthritis: a feasibility study in primary care. Pilot Feasibility Stud 2018; 4(1): 93-100. https://doi.org/10.1186/s40814-018-0288-2.
- Urits I, Jones M, Patel R, Adamian L, Seifert D, Thompson W. et al. Minimally Invasive Interventional Management of Osteoarthritic Chronic Knee Pain. J Knee Surg 2019; 32(1): 72-79. https://doi.org/10.1055/s-0038-1676071.
- Vakharia RM, Roche MW, Alcerro JC, Lavernia CJ. The Current Status of Cell-Based Therapies for Primary Knee Osteoarthritis. Orthop Clin North Am 2019; 50(4): 415-423. https://doi.org/10.1016/j.ocl2019.06.001.
- Deykalo VP, Mastyikov AN, Boloboshko KB. Platelet-rich plasma in the treatment of diseases and injuries of the musculoskeletal system. Vestnik of Vitebsk State Medical University 2011; 10(4): 6-12. Russian. https://elibrary.ru/item.asp?id=17255106.
- Panagos A. Resolution of a greater than 50-year history of severe, chronic low back pain following an ultrasound-guided platelet-rich plasma infiltration of the thoracolumbar fascia. Cureus 2018; 10(10): e3457. https://doi.org/10.7759/cureus.3457.
- Sonis AG, Sefedinova MY, Bezrukova MA, Marchenko AA, Ladonin SV. The use of platelet-rich autoplasma in the treatment of patients with pyoinflammatory diseases of soft tissues, bones and joints. Aspirantskij Vestnik Povolzh''ja 2016; (5-6):162-167. Russian. https://elibrary.ru/item.asp?id=28376160.
- Mohammed S, Yu J. Platelet-rich plasma injections: an emerging therapy for chronic discogenic low back pain. J Spine Surg 2018; 4(1): 115-122. https://doi.org/10.21037/jss.2018.03.04.
- Singla V, Batra YK, Bharti N, Goni VG, Marwaha N. Steroid vs. plateletrich plasma in ultrasound-guided sacroiliac joint injection for chronic low back pain. Pain Pract 2017; 17(6): 782-791. https://doi.org/10.1111/papr.12526.
- Kuffler DP. Platelet-rich plasma and the elimination of neuropathic pain. Mol Neurobiol 2013; 48(2): 315-332. https://doi.org/10.1007/s12035-013-8494-7.
- Benevolenskaya LI, Alekseeva LI. Diagnostic criteria of osteoarthrosis. Sovremennye problemy revmatologii. I congress of Russian rheumatologists. Orenburg, Russia, 1993: 191-192. Russian.
- Medvedev VL, Opolskiy AM, Kogan MI. Prospects for the development of regenerative technologies. Current knowledge of platelet rich plasma and the possibility of its application in treatment of complicated urological deseases. Kuban Scientific Medical Bulletin 2018; 25(3): 155-161. Russian. https://doi.org/10.25207/16086228-2018-25-3-155-161.
- Leushina EA, Filimonova OG, Chicherina EN, Betekhtina SN. Management of patients with osteoarthrosis and history of replacement arthroplasty: clinical case. Farmateka 2018; (S2): 75-79. Russian. https://doi.org/10.18565/pharmateca.2018.s2.75-79.
- Brandt KD, Fife RS, Braunstein EM, Katz B. Radiographic grading of the severity of knee osteoarthritis: relation of the Kellgren and Lawrence grade to a grade based on joint space narrowing, and correlation with arthroscopic evidence of articular cartilage degeneration. Arthritis Rheum 1991; 34(11): 1381-1386. https://doi.org/10.1002/art.1780341106.
- Karateev AE, Lila AM. Russian experience with injectable chondroitin sulfate and glucosamine sulfate: a review of clinical trials. Sovremennaya Revmatologiya 2018; 12(1): 33-40. Russian. https://doi.org/10.14412/1996-7012-2018-1-33-40.
- Clement ND, Weir DJ, Holland J, Deehan DJ. Contralateral knee pain reduces the rate of patient satisfaction but does not clinically impair the change in WOMAC score after total knee arthroplasty. Bone Joint J 2020; 102-B(1): 125-131. https://doi.org/10.1302/0301-620X.102B1.BJJ-2019-0328.R1.
- Hosseinzadeh A, Kamrava SK, Joghataei MT, Darabi R, Shakeri-Zadeh A, Shahriari M. et al. Apoptosis signaling pathways in osteoarthritis and possible protective role of melatonin. J Pineal Res 2016; 61(4): 411–425. https://doi.org/10.1111/jpi.12362.
- Tkachuk PV, Strafun SS, Kumchenko OB, Savosko SI, Gayevich IV, Makarenko OM. et al. Assessment of the effect of platelet plasma on blood biochemical parameters in an experimental model of knee osteoarthritis. Trauma 2019; 20(4): 39-45. Russian. https://doi.org/10.22141/1608-1706.4.20.2019.178744.
- Miller LE, Fredericson M, Altman RD. Hyaluronic Acid Injections or Oral Nonsteroidal Anti-inflammatory Drugs for Knee Osteoarthritis: Systematic Review and Meta-analysis of Randomized Trials. Orthop J Sports Med 2020; 8(1): 2325967119897909. https://doi.org/10.1177/2325967119897909.
- Bannuru RR, Natov NS, Dasi UR, Schmid CH, McAlindon TE. Therapeutic trajectory following intra-articular hyaluronic acid injection in knee osteoarthritis – meta-analysis. Osteoarthritis Cartilage 2011; 19(6): 611-619. https://doi.org/10.1016/j.joca.2010.09.014.
- Can VC, Locke IC, Kaneva MK, Kerrigan MJP, Merlino F, De Pascale C. et al. Novel anti-inflammatory and chondroprotective effects of the human melanocortin MC1 receptor agonist BMS-470539 dihydrochloride and human melanocortin MC3 receptor agonist PG-990 on lipopolysaccharide activated chondrocytes. Eur J Pharmacol 2020; 872: 172971. https://doi.org/10.1016/j.ejphar.2020.172971.
- Zhang X, Yao J, Wu Z, Zou K, Yang Z, Huang X. et al. Chondroprotective and antiarthritic effects of Daphnetin used in vitro and in vivo osteoarthritis models. Life Sci 2020; 240: 116857. https://doi.org/10.1016/j.lfs.2019.116857.
- Sampson S, Gerhardt M, Mandelaum B. Platelet rich plasma injection grafts for musculoskeletal injuries: A review. Curr Rev Musculoskelet Med 2008; 1(3-4): 165-174. https://doi.org/10.1007/s12178-008-9032-5.
- Wu W, Chen F, Liu Y, Ma Q, Mao T. Autologous injectable tissue-engineered cartilage by using platelet-rich plasma: experimental study in a rabbit model. J Oral Maxillofac Surg 2007; 65(10): 1951-1957. https://doi.org/10.1016/j.joms.2006.11.044.
- Anitua E, Sánchez M, Nurden AT, Zalduendo MM, de la Fuente M, Azofra J. et al. Platelet-released growth factors enhance the secretion of hyaluronic acid and induce hepatocyte growth factor production by synovial fibroblasts from arthritic patients. Rheumatology (Oxford) 2007; 46(12): 1769-1772. https://doi.org/10.1093/rheumatology/kem234.
- Chahla J, Cinque ME, Piuzzi NS, Mannava S, Geeslin AG, Murray IR. et al. A call for standardization in platelet-rich plasma preparation protocols and composition reporting: a systematic review of the clinical orthopaedic literature. J Bone Joint Surg Am 2017; 99(20): 1769-1779. https://doi.org/10.2106/jbjs.16.01374.
- Boden AL, Scott MT, Dalwadi PP, Mautner K, Mason RA, Gottschalk MB. Platelet-rich plasma versus Tenex in the treatment of medial and lateral epicondylitis. J Shoulder Elbow Surg 2019; 28(1): 112-119. https://doi.org/10.1016/j.jse.2018.08.032.
Received 4 August 2020, Revised 9 December 2020, Accepted 12 February 2021
© 2020, Basnaev U.I., Karakursakov N.E., Mykhaylichenko V.Yu., Kriventsov M.A.
© 2020, Russian Open Medical Journal
Correspondence to Usein I. Basnaev. E-mail: vivausein@gmail.com.