Introduction
Atrial fibrillation (AF) and chronic heart failure (CHF) often go together, thus aggravating the course of each other. The development of AF doubles the mortality of CHF patients, while the development of CHF triples mortality in patients with AF [1].
Transition from paroxysmal atrial fibrillation to a permanent form is often characterized by the structural remodeling progression in atria [2, 3]. Underlying diseases may cause chronic stretch and atrial dilatation, which appear important stimuli for chronic atrial structural remodeling (cellular hypertrophy, fibroblast proliferation, and tissue fibrosis), thereby enabling the maintenance of AF [4]. An increased left atrial (LA) size is a well-established, independent predictor of AF [5, 6].
The pulmonary veins (PV) constitute 90% source of ectopic beats initiating PAF and respond well to PV-directed ablation procedures [7]. Some studies, viz., those using magnetic resonance imaging [8], multidetector computed tomography, intracardiac echocardiography [9], and cineangiography, have shown PV dilatation in AF and the predicative role of pulmonary veins [10-12]. In theory, the pulmonary vein can enlarge as a result of the left atrium remodeling with increasing pressure in it; the LA and the left ventricle (LV) being separated only by PV muscle sleeves. The LA remodeling with increasing pressure in it occurs as a result of diastolic dysfunction of the left ventricle [13]. However, the literature presents cases of PV dilatation detected by means of 3 dimensional computed tomography before the increase of left atrial indexed volume (LAVI) measured via transthoracic echocardiography, thereby declaring the priority of PV remodeling before a change in the left atrium (LA) [10].
Patients with AF having chronic heart failure are presented to show an increased content of total fluid and extracellular fluid in the body measured with the use of bioimpedance vector analysis; the increased content is most pronounced in permanent AF [14, 15].
We have previously detected the presence of venous pulmonary hypertension due to the enlargement of the maximum and minimum diameters of pulmonary veins in CHF patients. We propose to discuss the function of fluid retention as one of the components of LA and PV enlargement in ventricular diastolic dysfunction. Published sources described a large number of patients with AF without diagnosed CHF despite the presence of cardiovascular disease in their anamneses [16, 11]; no congestion phenomena in the circulations were diagnosed in the patients; therefore, diuretic therapy was not prescribed for them. As noted above, a small number of CHF patients with paroxysmal AF exhibited termination of AF attacks within a year of observation when a loop diuretic, specifically, torasemide, was prescribed in a dose of 10 to 30 mg [17].
The objective of our study was to perform a transthoracic echocardiographic study of the left atrium remodeling and the maximum and minimum diameters of pulmonary veins, as well as remodeling regression during heart failure treatment, in patients with CHF and different forms of atrial fibrillation.
Material and methods.
Patients
The study prospectively recruited 299 patients with chronic heart failure and 132 virtually healthy people aged 35.08±1.80 (control group), examined in Uralskaya Clinic, LLC (Ekaterinburg, Russia). Patients with CHF were divided into three groups: group 1 (n=225) included patients with chronic heart failure without atrial fibrillation; group 2 (n=38) consisted of patients having chronic heart failure with symptomatic paroxysmal atrial fibrillation; group 3 (n=36) was represented by patients with chronic heart failure and symptomatic permanent AF. Chronic heart failure was diagnosed sensu the latest recommendations on CHF diagnostics [18]. The inclusion criteria were at least two episodes of symptomatic atrial fibrillation within the preceding 6 months but no AF episode longer than 7 days (without spontaneous termination or cardioversion). The exclusion criteria were patients with secondary atrial fibrillation (due to cardiac surgery, infection, or hyperthyroidism) and patients who underwent radiofrequency ablation.
The informed consent for research was obtained from all the patients. All the patients gave a written consent for participation in the study, which was approved by the local ethic committee of the Institute of Medical Cell Technologies (Ekaterinburg, Russia).
Echocardiographic examination
Transthoracic echocardiography was recorded using a Philips HD-15 device (USA) according to the standard protocol with the additional determination of the maximum and minimum diameters of the visualized pulmonary vein in order to diagnose pulmonary venous hypertension [19] at the beginning of the study and after six months of treatment including background CHF therapy and a loop diuretic, namely torasemide.
Statistical analyses
The use of the chi-square test showed that the available sample met the criteria for a normal distribution. Statistical analysis of the research results was conducted via Student’s t-test. Statistically significant differences were considered at p <0.05. The data were presented in the form M±SD, where M is the average value of the measured values, SD is standard deviation.
Results
The CHF patients with paroxysmal AF and permanent AF were elder than those without AF (Table 1). The patients with different forms of AF did not differ in age. The groups were comparable in terms of associated pathologies. In the group of patients with permanent AF, male gender prevailed, compared to other groups, probably, due to their late medical consultation. Table 1 presents a general characteristic of the patients.
Table 1. Characteristic of CHF patients with permanent atrial fibrillation, with paroxysmal one, and without atrial fibrillation
|
Parameter |
Control values (n=132) |
CHF patients without AF (n=225) |
Р |
Patients with paroxysmal AF (n=38) |
Р |
Р' |
Patients with permanent AF (n=36) |
Р |
Р' |
Р'' |
|
Gender (male). % |
30.3 |
27.5 |
0.788 |
18.4 |
0.225 |
0.349 |
50 |
0.076 |
0.046 |
0.002 |
|
Age. years |
35.08±9.86 |
65.01±10.37 |
0.001 |
70.31±8.92 |
0.001 |
0.002 |
69.28±10.07 |
0.001 |
0.030 |
0.750 |
|
Arterial hypertension. % |
0 |
100 |
|
100 |
|
|
100 |
|
|
|
|
Coronary artery disease. % |
0 |
12.9 |
≤0.001 |
10.5 |
0.042 |
0.662 |
11.1 |
0.041 |
0.754 |
0.934 |
|
Type 2 diabetes mellitus. % |
0 |
4.9 |
≤0.001 |
10.5 |
0.042 |
0.287 |
8.3 |
0.080 |
0.485 |
0.747 |
|
Acute stroke. % |
0 |
4 |
≤0.001 |
2.6 |
0.321 |
0.631 |
5.6 |
0.153 |
0.695 |
0.521 |
|
AH duration. years |
0 |
14.95±10.91 |
|
25.86±12.22 |
|
<0.001 |
26.50±12.32 |
|
<0.001 |
0.823 |
|
AF duration from the first attack. years |
0 |
0 |
|
2.88±1.69 |
|
|
9.69±4.50 |
|
|
<0.001 |
|
Inferolateral wall of the left ventricle. mm |
7.73±0.89 |
11.20±2.08 |
≤0.001 |
10.95±1.91 |
≤0.001 |
0.461 |
11.50±1.86 |
≤0.001 |
0.377 |
0.210 |
|
Interventricular septum thickness. mm |
8.11±1.21 |
12.18±2.57 |
≤0.001 |
12.11±2.36 |
≤0.001 |
0.901 |
12.27±1.76 |
≤0.001 |
0.803 |
0.820 |
|
Inferior wall of the right ventricle. mm |
4.22±3.94 |
7.95±5.41 |
≤0.001 |
7.34±1.23 |
≤0.001 |
0.461 |
8.88±3.73 |
≤0.001 |
0.194 |
0.035 |
|
Myocardial mass index. g/m2 |
82.35±15.85 |
107.93±27.25 |
≤0.001 |
108.63±22.37 |
≤0.001 |
0.900 |
105.19±25.82 |
≤0.001 |
0.556 |
0.509 |
|
Right atrium volume. ml |
33.69±5.84 |
53.74±16.30 |
≤0.001 |
54.14±15.36 |
≤0.001 |
0.883 |
101.21±35.76 |
≤0.001 |
≤0.001 |
≤0.001 |
|
Left atrium in the left lateral position. mm |
29.57±3.70 |
38.60±6.43 |
≤0.001 |
41.26±4.36 |
≤0.001 |
0.003 |
46.35±5.60 |
≤0.001 |
≤0.001 |
≤0.001 |
|
Left atrium volume. ml |
42.94±7.89 |
85.51±22.83 |
≤0.001 |
103.41±31.19 |
≤0.001 |
0.002 |
124.65±17.78 |
≤0.001 |
≤0.001 |
0.006 |
|
LAVI |
23.64±2.56 |
44.28±9.58 |
≤0.001 |
58.81±17.37 |
≤0.001 |
≤0.001 |
67.16±17.78 |
≤0.001 |
≤0.001 |
0.049 |
|
Left ventricular end-diastolic volume. ml |
93.31±22.60 |
107.56±43.58 |
≤0.001 |
109.38±33.85 |
0.006 |
0.771 |
110.62±40.22 |
0.013 |
0.675 |
0.930 |
|
Left ventricular end-systolic volume. ml |
29.94±9.07 |
35.35±21.95 |
≤0.001 |
33.52±12.63 |
0.106 |
0.470 |
48.21±28.88 |
≤0.001 |
0.014 |
0.009 |
|
Stroke volume. ml |
62.88±15.36 |
72.54±25.84 |
≤0.001 |
74.41±19.22 |
0.001 |
0.603 |
62.41±16.97 |
0.879 |
0.004 |
0.009 |
|
Simpson’s ejection fraction. % |
66.67±5.19 |
67.28±6.79 |
0.343 |
68.71±4.24 |
0.014 |
0.091 |
58.38±11.58 |
≤0.001 |
≤0.001 |
≤0.001 |
|
Systolic pressure in the pulmonary artery. mm Hg |
14.74±5.26 |
23.43±8.86 |
≤0.001 |
26.05±9.55 |
≤0.001 |
0.124 |
34.82±9.68 |
≤0.001 |
≤0.001 |
≤0.001 |
|
IVC. mm |
18.83±2.85 |
17.13±3.14 |
≤0.001 |
16.79±2.92 |
≤0.001 |
0.514 |
19.75±2.90 |
0.071 |
≤0.001 |
≤0.001 |
|
TAPSE. mm |
22.43±2.29 |
22.19±3.82 |
0.453 |
21.40±3.04 |
0.060 |
0.166 |
16.50±4.10 |
≤0.001 |
≤0.001 |
≤0.001 |
|
Average Е/e’ |
6.26±1.52 |
10.03±3.34 |
≤0.001 |
11.34±5.32 |
≤0.001 |
0.149 |
14.78±4.19 |
≤0.001 |
≤0.001 |
0.003 |
|
Maximum pulmonary vein diameter. mm |
13.51±0.91 |
20.64±2.90 |
≤0.001 |
22.21±2.03 |
≤0.001 |
≤0.001 |
23.91±3.08 |
≤0.001 |
≤0.001 |
0.002 |
|
Minimum pulmonary vein diameter. mm |
5.7±0.49 |
10.51±2.96 |
≤0.001 |
13.15±3.13 |
≤0.001 |
≤0.001 |
15.10±3.07 |
≤0.001 |
≤0.001 |
0.010 |
The patients with chronic heart failure, regardless of the absence or presence of paroxysmal or permanent atrial fibrillation, had structural changes in the heart, compared with the control group, which were detected via echocardiography (Table 1).
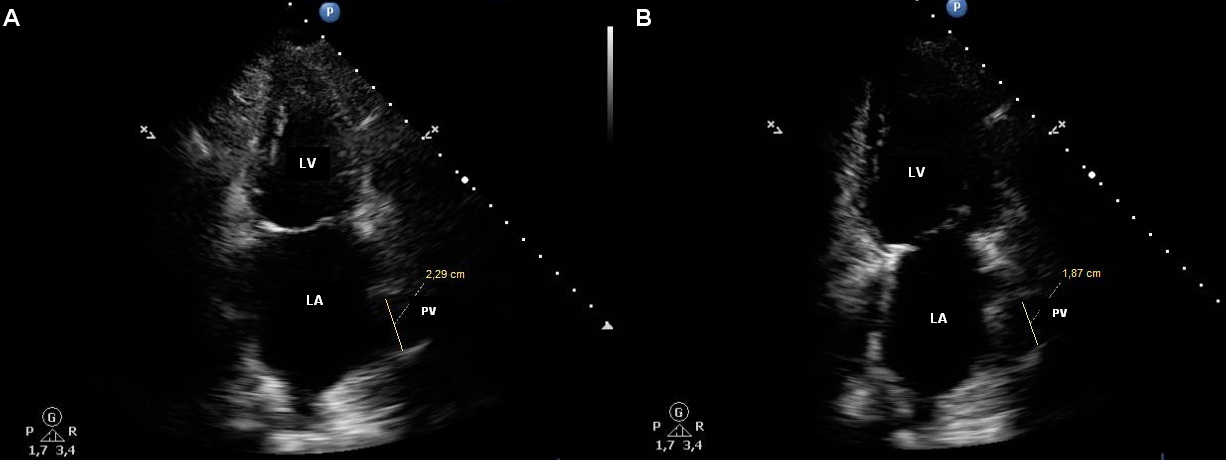
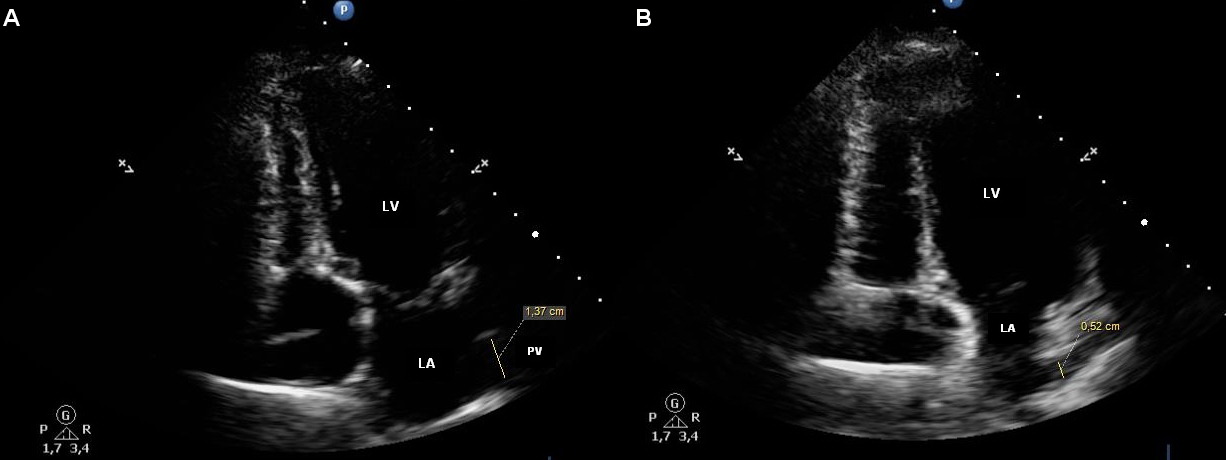
The patients with chronic heart failure having paroxysmal or permanent atrial fibrillation, compared to patients without AF, had significantly more distinct structural changes in their heart. The latter were as follows: more obvious LA dilatation (left atrium volume of 103.41±31.19, 124.65±17.78, 85.51±22.83 ml; LAVI of 58.81±17.37, 67.16±17.78, and 44.28±9.58), dilatation of the left lower pulmonary vein of the maximum (22.21±2.03, 23.91±3.08, and 20.64±2.90 mm) and minimum (13.15±3.13, 15.10±3.07, and 10.51±2.96 mm) diameters (Table 1). Figures 1А, 2А, 3А, and 4А demonstrate maximum PV diameter in the patients with chronic heart failure without AF, in the patients having paroxysmal and permanent AF, and in the control subjects; Figures 1В, 2В, 3В, and 4В show their minimum PV diameter.
Figure 1. Pulmonary vein in a patient having CHF without AF. A. The maximum diameter of the pulmonary vein (20.0 mm). B. The minimum diameter of the pulmonary vein (10.1 mm).
Figure 2. Pulmonary vein in a patient with CHF and paroxysmal AF. A. The maximum diameter of the pulmonary vein (21.3 mm). B. The minimum diameter of the pulmonary vein (14.7 mm).
Figure 3. Pulmonary vein in a patient with CHF and permanent AF. A. The maximum diameter of the pulmonary vein (22.9 mm). B. The minimum diameter of the pulmonary vein (18.7 mm).
Figure 4. Pulmonary vein in a healthy person without cardiovascular pathology. A. The maximum diameter of the pulmonary vein (13.7 mm). B. The minimum diameter of the pulmonary vein (5.2 mm).
We detected differences in myocardial remodeling in the patients with different forms of atrial fibrillation. The patients with permanent AF, compared to the patients with paroxysmal AF, had larger LA dilatation and larger dilatation of the left lower pulmonary vein of the maximum and minimum diameters with higher average E/e′ (Table 1). Besides, the dilatation of right atrium, more evident LV myocardial hypertrophy, in combination with increased systolic pressure in the pulmonary artery at a lower LV preserved ejection fraction and a decreased systolic function of the right ventricle (Table 1), were detected in the CHF patients with permanent atrial fibrillation.
After 6 months of standard CHF therapy with individual selection of a loop diuretic, the following significant improvements in echocardiographic indices were detected both in the group of patients with paroxysmal and permanent AF (Table 2): the left atrial volume index decreased from 58.81±17.37 to 43.00±3.70 (р≤0.001) and from 67.16±17.78 to 58.53±22.37 (р=0.085), respectively; the maximum diameter of the pulmonary vein decreased from 22.21±2.03 mm to 19.70±1.00 mm (р≤0.001) and from 23.91±3.08 mm to 21.77±3.21 mm (р=0.008), respectively; the minimum diameter of the pulmonary vein declined from 13.15±3.13 mm to 10.22±0.63 mm (р≤0.001) and from 15.10±3.07 mm to 12.34±2.13 mm (р≤0.001), respectively, this being an indication of reduced pressure in the pulmonary veins.
Table 2. Echocardiography of the LA and the PV in patients with paroxysmal AF and permanent AF before and 6 months after therapy correction
|
Parameter |
Patients with paroxysmal AF (n=38) |
|
Patients with permanent AF (n=36) |
|
||
|
Before therapy correction |
After therapy correction |
Р |
Before therapy correction |
After therapy correction |
Р |
|
|
LAVI |
58.81±17.37 |
43.00±3.70 |
<0.001 |
67.16±17.78 |
58.53±22.37 |
0.085 |
|
Maximum diameter of the pulmonary vein. mm |
22.21±2.03 |
19.70±1.00 |
<0.001 |
23.91±3.08 |
21.77±3.21 |
0.008 |
|
Minimum diameter of the pulmonary vein. mm |
13.15±3.13 |
10.22±0.63 |
<0.001 |
15.10±3.07 |
12.34±2.13 |
<0.001 |
|
Therapy |
|
|
|
|
|
|
|
Beta blockers. % |
40 |
100 |
<0.001 |
70.6 |
100 |
<0.001 |
|
ACEIs/ARBs. % |
20/60 |
13.3/86.7 |
0.436/0.009 |
23.5/58.8 |
29.4/70.6 |
0.573/0.300 |
|
Indapamide/HCTZ or chlorthalidone/torasemide. % |
20/6.7/0 |
0/0/100 |
0.004/0.107/0 |
59/5.9/0 |
0/0/100 |
<0.001/0.142/0 |
|
Spironolactone or eplerenone/furosemide. % |
26.7/0 |
26.7/0 |
1/0 |
23.5/5.9 |
52.9/0/0 |
0.011/0.142 |
|
Calcium-channel blockers. % |
40 |
0 |
<0.001 |
11.8 |
11.8 |
1 |
|
Antyarrhythmics |
13.3 |
0 |
0.021 |
0 |
0 |
1 |
Paroxysms stopped as a result of therapy in patients with paroxysmal AF; no paroxysms appeared within 1 year of observation.
Discussion
The transthoracic echocardiographic study demonstrated that the patients with chronic heart failure having atrial fibrillation had more evident structural impairments of the heart walls and chambers, compared to those in the patients without arrhythmia; this manifested itself in a more pronounced dilatation of the left atrium, with a pulmonary vein flowing into it. It was also shown that the patients with chronic heart failure and permanent atrial fibrillation had more obvious structural impairments of the heart chambers and hemodynamics, compared to those in the patients with paroxysmal atrial fibrillation; this manifested itself in a more pronounced dilatation of the left atrium, with a pulmonary vein flowing into it, and a higher value of the average E/e′ ratio. The CHF patients with permanent AF had a dilatation of the right atrium with greater hypertrophy of the RV inferolateral wall, along with increased systolic pressure in the pulmonary artery and the RV systolic dysfunction estimated according to TAPSE. Other researchers also noted that both left atrium and the right atrium possess structural features contributing to the pathogenesis of AF [20].
The prescription of diuretic therapy, viz., torasemide at a dosage of 10 to 30 mg per day, for the patients with paroxysmal AF and the permanent AF resulted in a significant decrease in LAVI in the patients with paroxysmal AF and a reduction of the maximum and minimum diameters of PV in the patients with paroxysmal AF and permanent AF, the measurements made via dynamic transthoracic echocardiography in six months. Besides, AF attacks stopped within a year of observation. Before the therapy was corrected, 40% of the patients had been treated by basic medications for CHF, viz., by beta-blockers and angiotensin converting enzyme inhibitors or angiotensin receptor blockers. However, when visiting a physician, the patients complained of having AF attacks; this implied the role of the diuretics facilitating the reduction of LA and PV in terminating the paroxysms.
It is known from the literature sources that the use of renin–angiotensin blockers and diuretics is associated with a reduced AF development after atrial flutter ablation [21].
Although the study followed more than one group of patients with arterial hypertension, who developed paroxysmal AF, later on transforming into permanent AF, it indirectly indicated that CHF development with fluid congestion in the pulmonary circulation, followed by the enlargement of the LA and the PV, was a basis for AF formation and the transformation of paroxysmal AF into permanent AF in patients with prolonged AH in their medical histories.
Limitations
The sample size of participants was small. The patients under study were not the same in the groups, and this limited our understanding of the actual remodeling of left atrial myocardium and pulmonary vein myocardium in the course of AF formation and paroxysmal AF transformation into permanent AF.
Some of the patients had an atypical position of the pulmonary veins [20], and this could limit the visualization of pulmonary veins and the measurement of their diameters. The limitations of heart visualization by echocardiography were also among the limitations of the proposed method.
The study including a loop diuretic prescribed for the patients with paroxysmal AF who exhibited the termination of paroxysms was not randomized; consequently, additional research of the kind is needed.
Conclusion
Echocardiography proved its applicability to measuring the maximum and minimum PV diameters in patients with CHF.
The LA and the maximum and minimum PV diameters enlarge in proportion to the development of paroxysmal AF in patients with CHF and the transformation of paroxysmal AF into permanent AF. Thus, more significant dilatation of the left atrium with pulmonary veins flowing into it, with their increasing dilatation in association with paroxysmal AF, and its transformation into permanent AF, has been detected in patients with chronic heart failure and atrial fibrillation. In permanent AF, besides changes in the left chambers, there were changes in the right chambers.
The normalization of the PV diameter in patients with CHF and paroxysmal AF resulted in the termination of arrhythmia attacks.
Funding
This study was funded partly by the state assignment to the USMU of the Ministry of Health of the Russian Federation for 2021–2023, No. 121030900298-9. Topic: Individualization of Selecting Complex Anti-Aging Therapy.
Conflict of interest
The author declare that they have no conflicts of interest.
- Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation 2003; 107(23): 2920-2925. https://doi.org/10.1161/01.cir.0000072767.89944.6e.
- Nattel S, Guasch E, Savelieva I, Cosio FG, Valverde I, Halperin JL, et al. Early management of atrial fibrillation to prevent cardiovascular complications. Eur Heart J 2014; 35(22): 1448-1456. https://doi.org/10.1093/eurheartj/ehu028.
- de Vos CB, Pisters R, Nieuwlaat R, Prins MH, Tieleman RG, Coelen RJ, et al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol 2010; 55(8): 725-731. https://doi.org/10.1016/j.jacc.2009.11.040.
- Eckstein J, Verheule S, de Groot NM, Allessie M, Schotten U. Mechanisms of perpetuation of atrial fibrillation in chronically dilated atria. Prog Biophyss Mol Biol 2008; 97(2-3): 435-451. https://doi.org/10.1016/j.pbiomolbio.2008.02.019.
- Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol 1998; 82(8A): 2N-9N. https://doi.org/10.1016/s0002-9149(98)00583-9.
- Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation 1994; 89(2): 724-730. https://doi.org/10.1161/01.cir.89.2.724.
- Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998; 339(10): 659-666. https://doi.org/10.1056/nejm199809033391003.
- Tsao HM, Yu WC, Cheng HC, Wu MH, Tai CT, Lin WS, et al. Pulmonary vein dilation in patients with atrial fibrillation: Detection by magnetic resonance imaging. J Cardiovasc Electrophysiol 2001; 12(7): 809-813. https://doi.org/10.1046/j.1540-8167.2001.00809.x.
- Schwartzman D, Lacomis J, Wigginton WG. Characterization of left atrium and distal pulmonary vein morphology using multidimensional computed tomography. J Am Coll Cardiol 2003; 41(8): 1349-1357. https://doi.org/10.1016/s0735-1097(03)00124-4.
- Kim S, Kim YH, Lee SH, Kim JS. Pulmonary Vein Enlargement as an Independent Predictor for New-Onset Atrial Fibrillation. J Clin Med 2020; 9(2): 401. https://doi.org/10.3390/jcm9020401.
- Kurata M, Asano T, Mori H, Mase H, Nagumo S, Wakatsuki D, et al. Can an increase in the pulmonary vein volume measured by three dimensional computed tomography predict the presence of atrial fibrillation? J Arrhythm 2019; 35(2): 230-237. https://doi.org/10.1002/joa3.12158.
- Lu YY, Chen YC, Lin YK, Chen SA, Chen YJ. Mechanoelectrical feedback in pulmonary vein arrhythmogenesis: Clinical challenges and therapeutic opportunities. J Arrhythm 2020; 36(4): 608-614. https://doi.org/10.1002/joa3.12391.
- Tsang TS, Gersh BJ, Appleton CP, Tajik AJ, Barnes ME, Bailey KR, et al. Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J Am Coll Cardiol 2002; 40(9): 1636-1644. https://doi.org/10.1016/s0735-1097(02)02373-2.
- Kirillova VV, Sokolova LA, Garganeeva AA. Fluid retention in patients with paroxysmal and permanent forms of atrial fibrillation and chronic heart failure. Eur J Heart Failure 2020; 22(Suppl. S1), 142-143. https://doi.org/10.1002/ejhf.1963.
- Kirillova VV, Sokolova LA, Garganeyeva AA, Meshchaninov VN, Batalov RE. Fluid retention in patients with paroxysmal and permanent atrial fibrillation and heart failure. Cardiovascular Therapy and Prevention 2020; 19(5): 2402. Russian https://doi.org/10.15829/1728-8800-2020-2402.
- Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Moretz K, et al. CABANA Investigators. Catheter Ablation versus Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA) Trial: Study Rationale and Design. Am Heart J 2018; 199: 192-199. https://doi.org/10.1016/j.ahj.2018.02.015.
- Kirillova VV. Method of treating paroxysmal form of atrial fibrillation in patients with chronic heart failure in arterial hypertension. The RF Patent for Invention. No. RU 2703517 C1. 2019. Russian. https://elibrary.ru/item.asp?id=41185135.
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37(27): 2129-2200. https://doi.org/10.1093/eurheartj/ehw128.
- Kirillova VV. Early ultrasound diagnostic venous pulmonary hypertension. Eur J Heart Failure 2017; 19(Suppl 1): 496. https://doi.org/10.1002/ejhf.833.
- Lin YJ, Tai CT, Kao T, Tso HW, Huang JL, Higa S, et al. Electrophysiological characteristics and catheter ablation in patients with paroxysmal right atrial fibrillation. Circulation 2005; 112: 1692-1700. https://doi.org/10.1161/CIRCULATIONAHA.104.512731.
- Anné W, Willems R, Van der Merwe N, Van de Werf F, Ector H, Heidbüchel H. Atrial fibrillation after radiofrequency ablation of atrial flutter: Preventive effect of angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, and diuretics. Heart 2004; 90(9): 1025-1030. https://doi.org/10.1136/hrt.2003.023069.
Received 30 April 2021, Revised 17 February 2022, Accepted 25 February 2022
© 2021, Russian Open Medical Journal
Correspondence to Venera V. Kirillova. Address: Department of Biochemistry, Ural State Medical University, 3 Repina St., Ekaterinburg 620028, Russia. Phone: +7912270 8496. Е-mail: venova@list.ru.