Introduction
Diabetes mellitus (DM) is a metabolic syndrome characterized by chronic hyperglycemia due to the disorder of insulin secretion or insulin sensitivity or both at the same time [1]. Hyperglycemia could lead to DM through a mechanism which increases the production of radical compounds, reactive oxygen species (ROS) and reactive nitrogen species (RNS). They overproduct without a sufficient antioxidant defense would cause oxidative stress conditions [2]. This condition causes vascular endothelial cell damage and results in macro and microangiopathy complications on DM [3].
Vascular endothelial cells act as a selective barrier to prevent the transfer of some substances on the vascular system. The structure of endothelial cells is composed of actin-based cytoskeleton which relates to its function as barrier permeability [4]. The endothelial barrier consists of an actin filament, stress fibers, junction-associated actin filament system, and adhesion molecule which maintain the permeability of the cells against solutes, solvents, hormones, and macromolecules [5]. Permeability of endothelial cells is also controlled by a signaling mechanism which regulates cellular activity such as calcium (Ca) ions, protein kinases, and phosphatases [6]. Human umbilical vein endothelial cells (HUVEC’S) culture was used in this study due to several advantages. The culture is an in vitro model of vascular endothelium that can be used to evaluate its structure and function. The use of this culture is better in representing the actual human condition, while reliable, reproducible, and effective both in time and cost [4].
ROS, produced in a hyperglycemic condition, could damage the structure and function of endothelial cells through oxidative stress [6]. ROS accumulation causes intercellular gaps, actin filaments disorganization, and the change of cell shape, therefore increasing the paracellular permeability [7]. ROS decreases trans-endothelial electric resistance and increases trans-endothelial permeability against plasma proteins such as albumin. Oxidative stress steps up the Ca level in the cytosol, which increases endothelial permeability through cell contraction, gap junction formation, and actin filament disorganization [8].
Antioxidant works to neutralize free radicals and reduce oxidative stress damage. Ascorbic acid and α-tocopherol are anti-oxidants that have different characteristics. α-tocopherol is a lipid-soluble antioxidant protecting cells membranes against free radical, whereas ascorbic acid is water soluble which works extracellularly [9, 10]. When antioxidant scavenges ROS, they result in radical substances and therefore increase oxidative stress condition [11]. The application of antioxidant combination is more recommended compared to a single antioxidant as it reduces more oxidative stress damage. It is expected to increase the efficacy and reduce the toxicity or adverse reaction [12]. As antioxidants, ascorbic acid and α-tocopherol have not been evaluated on endothelial permeability, especially when combined.
Aim of the study: To explore the effect of ascorbic acid, α-tocopherol and its combination on the permeability of HUVEC’S culture that was exposed to high glucose concentration.
Material and Methods
Chemical uses
Ascorbic acid, α-tocopherol, collagenase type II, glucose, Hank’s Balanced Salt Solution (HBSS), New Calf Serum (NCS), serum-free medium, trypsin Ethylenediaminetetraacetic acid (EDTA), phosphate buffer saline (PBS), and albumin labeled trypan blue. All chemicals were purchased from Sigma Aldrich (USA).
HUVEC’s culture
This research protocol was also approved by the institutional research ethical committee from the Faculty of Medicine Brawijaya University (ethical certificate No. 217/EC/KEPK-S3/03/2014), Malang Indonesia. Human umbilical vein endothelial cells (HUVECs) were obtained from the umbilical cord of neonates. Samples were collected based on inclusion criteria in some private hospital, Malang, after obtaining the informed consent. The procedure of HUVEC’s culture based on Jones 1996 with slight modifications. Endothelial cells were isolated by collagenase type II in 10 ml of serum-free media and incubated for 7 minutes. Next, the media solution was centrifuged in 1000rpm for 8 minutes. The pellet, containing endothelial cells, was collected for further culture isolation in 0.2% gelatin-coated flasks, which contain 4ml of M199 media. Then, the flask was stored in an incubator at 37°C and with 5% CO2. After the cell grew as monolayer, a passage or subculture step was performed using trypsin solution and the cells were transferred on to transwell polyester filter. Glucose (5 and 20 mM), ascorbic acid, and α–tocopherol was administrated concomitantly in culture media for seven days after the cells grew as monolayer. Single composition of both ascorbic acid and α–tocopherol was administrated each of them in three doses, 50 µM, 100 µM, 200 µM, respectively. To compare the effectivity, the antioxidant also combined under these following doses: I (25 µM: 25 µM), II (50 µM: 50 µM) and III (100 µM: 100 µM)
Barrier endothelial assay
HUVEC’s barrier assay was based on Lum and Roebuck, 2001 with slight modification. Endothelial permeability to albumin was assayed on two compartments separated by a filter membrane. Both of the compartments contain tyrode’s solution with slight modification by adding NCS 10 %. The luminal compartment contained endothelial cell on transwell polyester filter and 2.5 ml of tyrode’s solution, 0.2 ml albumin labeled with trypan blue 31,44 ppm was added in this compartment. Whereas the albuminal compartment contained 10 ml of tyrode’s solution and was continuously stirred with a magnetic stirrer. After 30 minutes, the solution in albuminal compartment was taken and measured for albumin labeled trypan blue level by spectrophotometer with λ=592 nm. Based on the previous study, the permeability assay was performed on the seventh day [6].
Histopathology of endothelial cells
Endothelial cells (HUVEC’s) culture was observed to characterize its morphology (such as: shape and size) and countable cells under a light microscope (M=400x).
Statistical analysis
All data are expressed as mean ± SD (standard deviation). Data was statistically tested using homogeneity and normality test before the sequel statistical test was performed. If the data is normal and homogeneous, the statistical analysis of the data was performed by One Way ANOVA test. Statistical test followed by a Dunnet test (p < 0.05) using SPSS (IBM).
Results
Effect of antioxidant ascorbic acid, α-tocopherol and its combination on the permeability of HUVEC’s culture
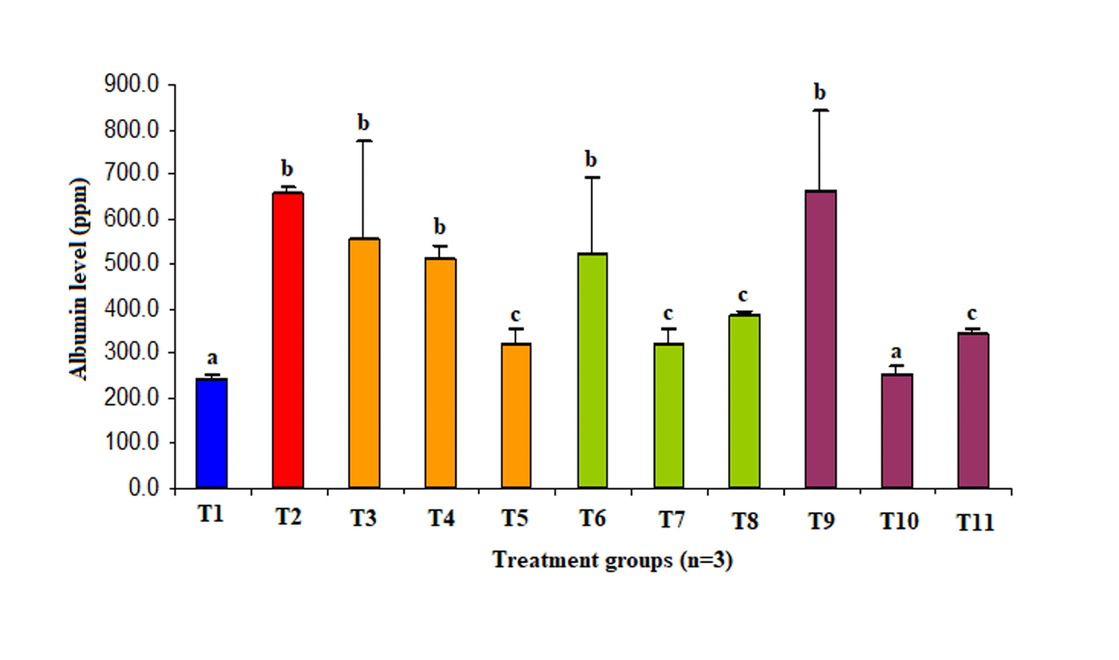
Barrier permeability of HUVEC’s culture exposed by normal or high concentration glucose and antioxidant are shown in Figure 1.
Figure 1. Potency ascorbic acid, α-tocopherol and its combination on permeability of HUVECS culture exposed high concentration glucose.
T1, glucose 5 µM; T2, glucose 22 µM; T3, glucose 22 µM + vit C 50 µM; T4, glucose 22 µM + vit C 100 µM; T5, glucose 22 µM + vit C 200 µM; T6, glucose 22 µM + vit E 50 µM; T7, glucose 22 µM + vit C 100 µM; T8, glucose 22 µM + vit C 200 µM; T9, glucose 22 µM + vit C 25 µM + vit E 25 µM; T10, glucose 22 µM + vit C 50 µM + vit E 50 µM; T11, glucose 22 µM + vit C 100 µM + vit E 100 µM.
Ascorbic acid 200 µM, α-tocopherol 100 µM, and 200 µM decreased endothelial permeability to 50%, 50%, and 40% respectively better than control group (p<0.05). Meanwhile, the combination of ascorbic acid and α-tocopherol 1 (50 µM: 50 µM) and α-tocopherol II (100 µM: 100 µM) reduced barrier permeability of endothelial to 60% and 50% respectively better than control group (p<0.05). Administration of the second combination of antioxidant decreased endothelial permeability until no difference to the normal group was observed (p>0.05). Moreover, HUVEC’s culture exposed to high concentration glucose increased its permeability three folds compared to normal concentration (p<0.05).
Effect of ascorbic acid, α-tocopherol and its combinations on the structure of HUVEC’s culture exposed high glucose concentration
Morphology of HUVEC’s culture exposed normal or high concentration glucose and antioxidant are shown in Figure 2.
Figure 2. Potency ascorbic acid, α-tocopherol and its combination on structure of HUVEC’s culture exposed high concentration glucose (M=200x). (A) 5 µM glucose: polygonal/cobblestone-shaped cells and fine-looking cell membranes/smoothing. (B) glucose 22 µM: cells undergoing apoptosis characterized by shrinkage cells and nucleus condensation. (C) glucose 22 µM + vit C 50 µM + vit 50 μM: cells and cell membranes do not show structural damage. (D) glucose 22 µM + vit E 100 μM; the cell membrane is fine and the polygonal cells do not appear to be structural damage. (E) glucose 22 µM + vit C 100 μM: cell membrane is not smooth due to membrane lipoprotein damage. (F) G22 + VITC.25 + VIT E. 25 μM: the cells undergo apoptosis and the nucleus is condensed.
HUVEC’s culture exposed to glucose at normal concentrations showed monolayer cells with a cobblestone shape and smooth cell membrane. Cell apoptosis was observed on high concentration glucose, characterized by cell shrinkage and nucleus condensation. Ascorbic acid, α-tocopherol, and its combination had more potential to protect cells against oxidative damage on endothelial cells exposed high concentration glucose.
Discussion
Effect of high concentration glucose expose on permeability and morphology of HUVEC’s culture
The exposure of high concentration glucose on HUVEC’s culture increased cell permeability. Glucose in high concentrations is oxidized into reactive dicarbonyl sugar which could bind to amino-lipids and proteins of endothelial cells, such as Vascular endothelial (VE)-cadherin [8]. Glycation of protein can destroy VE-cadherin structures, make they lose their elasticity and function as an adhesion molecule, resulting in barrier function impairment and detachment of endothelial cells [13]. Based on Figure 2, a high concentration of glucose could increases cell apoptosis rate, indicated by shrinkage and nucleus condensation, which increase endothelial permeability. Exposure of glucose at high concentration increases hydrogen peroxide radicals and Ca cytosol level, the highest level of both occurs at the seventh days [14, 15, 24]. The increase of cytosolic Ca levels activates phospholipase-C and results in myosin light chain kinase (MLCK) phosphorylation which initiates cytoskeleton contraction and increases endothelial permeability [6]. On the seventh days of treatment, hydrogen peroxide radical disrupts actin filament which is signaled by an increase of actin depolymerization (G-actin) controlling endothelial barrier function [16, 17, 25].
HUVEC’s culture showed increase cell permeability higher than normal cells. The morphology of normal endothelial cells is a monolayer, polygonal (cobblestone) shape and smooth cell membrane. Based on observation after staining process using anti-actin antibodies, it showed actin filament to be well arranged. On the other hand, the HUVEC’s culture has disorganization of actin filament [16, 25]. This structure is correlated with the barrier function, in which HUVEC’s permeability exposed to glucose at normal concentration was lower than the high concentration of glucose. Hyperglycemia condition is analog with high concentration glucose exposure which increases anion superoxide and protein kinase C (PKC) activation [9, 17]. Increase of PKC induces nicotinamide adenine dinucleotide phosphate (NADPH) oxidase to produce superoxide radicals and stimulate expression of endothelial adhesion molecule resulting in the dysfunction of endothelial barrier [17, 18]
Effect of antioxidant ascorbic acid on permeability and morphology of HUVECS culture
Ascorbic acid 200 µM reduces endothelial cells permeability if exposed to high concentration glucose. Ascorbic acid is a general ROS and RNS scavenging antioxidant at liquid phase, especially in the cytosol. Therefore ascorbic acid protects cells from damage caused by free radicals [12]. These include hydroxyl radicals, peroxyl radicals, superoxide anions, nitrogen dioxides, and also non-radical species, such as oxygen singlets, nitroxide, and peroxynitrite [2, 12]. Ascorbic acid prevents nitrosation process due to free radicals on target molecules. It can neutralize ROS effect and therefore avoiding the damage of actin filament structure which controls the barrier function [16]. Ascorbic acid can also protect the VE-cadherin structure which is regulating endothelial permeability. As VE-cadherin produces a complex compound with α-catenin and is connected by the cytoskeleton, disconnection of the junction with endothelial cells can be prevented [4, 20]. The previous study showed that ascorbic acid inhibited malondialdehyde (MDA) production and retained endothelial-derived relaxing factor (EDRF) and the level of endothelial nitric oxide synthase [26]. Ascorbic acid is an effective antioxidant scavenger. Firstly, it is due to both ascorbic acid and ascorbic radical have a low reduction potential, so they could react with all of radical and biology oxidants. Secondly, ascorbic radical has a low reactivity due to resonance stability on a free electron [11].
Ascorbic acid is entering the cells via two transporter systems, which are Na dependent transporter and glucose transporter-1 (GLUT-1). Disorders of the transporter system result in a decrease of antioxidant defense in intracellularly. The structure of dehydroascorbic (DHA) is similar to glucose, therefore competes to be transported by GLUT-1 into the cytoplasm [21]. Differences in physical and chemical properties of ascorbic acid can affect their potency as antioxidants in decreasing endothelial permeability when exposed to high concentration glucose [12]. Increasing the dosage of ascorbic acid up to 200 µM results in antioxidant entered into intracellularly after competing with glucose at 22 µM, and therefore this dose shows a real effect in decreasing HUVEC’s permeability exposed to high concentration glucose. The morphology of cells showed cells membrane to be smooth and no damage to cells membrane was found.
Ascorbic acid 50 µM and 100 µM did not significantly decrease endothelial permeability. Low doses of ascorbic acid cannot protect cells against oxidative damage and therefore resulting in the disorder of endothelial permeability. This was signaled by the passage number of albumins which was high in the albuminal compartment. On the other hand, low dose ascorbic acid cannot be transported to intracellularly effectively due to its failure to compete with high concentration glucose [12].
Effect of α-tocopherol on permeability and morphology of HUVECS culture exposed a high concentration of glucose.
α-tocopherol 100 µM and 200 µM decreases endothelial permeability after exposed to high concentration glucose. Antioxidant α-tocopherol works by inhibiting lipid peroxidation and lipoprotein damage on cells membrane especially at the lipid phase. α-tocopherol is a lipid-soluble antioxidant working in the lipid bilayer of cells membrane and functions by stopping chain reactions by scavenging peroxyl and inhibiting free radical production such as oxygen singlet on the cell membrane. Therefore lipoproteins can be protected against oxidation [2, 10]. α-tocopherol as an extracellular antioxidant can neutralize the effects of ROS and protects cell membrane against free radical. Eventually, the damage of actin filament and VE-cadherin that controlling the endothelial barrier function could be avoided [19]. α-tocopherol prevents lipid peroxidation on Poly Unsaturated Fatty Acid (PUFA) of cells membranes and minimizes LDL oxidation. The administration of α-tocopherol on the HUVECS culture, which was exposed to a high level of glucose, showed the decrease of MDA level and maintenance of nitric oxide level [26]. Besides that, α-tocopherol regulates membrane fluidity, stabilizes membrane and inhibits 5-lypoksigenase activity [19]. Lipid peroxidation is initiated through hydrogen bisalilic on lipid by peroxyl radical resulting in lipid and peroxide radicals. Oxygen is the result in peroxyl lipid radical production and lipid peroxidation propagation through a chain reaction. α-tocopherol reduces lipid peroxidation through two mechanics, a chain bounding of lipid radical carrier or scavenging of peroxyl radical [2, 12].
α-tocopherol at a 100 µM dosage was stronger in inhibiting permeability increase compared to 200 µM, based on albumin concentration in the albuminal compartment. This is caused by α-tocopherol 100 µM having a higher activity in protecting the stability of cell membrane lipoproteins from damage due to ROS. Cell morphology showed that the cell’s membrane was smooth and no damaged to the cell was found (Figure 2). Increasing the dose of α-tocopherol to 200 µM precisely increases the endothelial permeability. When α-tocopherol acts as an antioxidant, it will be oxidized into tocopheryl and this radical form is physiologically converted into tocopherol [10]. Administration of α-tocopherol 200 µM possibly produces tocopheryl radical in a huge concentration which cannot be regenerated by the physiological mechanism. Tocopheryl radical can disrupt actin filaments and VE-cadherin structure which results in the dysfunction of the endothelial barrier. Combination with ascorbic acid is suggested in α-tocopherol usage, especially in high doses [12, 19].
A single administration of α-tocopherol and ascorbic acid at the same dose (100 µM) obtained a different effect on endothelial permeability. α-tocopherol was more potent in protecting the barrier function of endothelial cells exposed high concentration glucose compared to ascorbic acid. α-tocopherol can reach the lipid membrane; therefore, it can protect the membrane from lipid peroxidation. Following the previous study, α-tocopherol prevented MDA production as a result of lipid membrane peroxidation on HUVEC culture exposed to high glucose concentration [26]. α-tocopherol works through dual-action both of as a direct and indirect antioxidant. The direct antioxidant mechanism through inhibition of free radical and production of Advance Glycate End Product (AGE), stop chains reaction production whereas indirect antioxidant work by the inhibition of Protein Kinase C (PKC) activation [10]. α-tocopherol was more effective in inhibiting the increase of endothelial permeability compared to ascorbic acid. This is caused by α-tocopherol works through dual-action whereas ascorbic acid works by scavenging free radical only [12].
Effect of combination of ascorbic acid and α-tocopherol on HUVECS culture exposed high glucose concentration
Combination of ascorbic acid and α–tocopherol at dosage 50 µM and 100 µM reduced the permeability of endothelial cells exposed high concentration glucose. The use of ascorbic acid and α–tocopherol combination results in a pharmacodynamic interaction and a synergistic effect to protect cells against free radicals. Moreover, the combination of antioxidants aims to reduce the doses of both of them and therefore adverse reaction can be minimized [23, 24]. The antioxidant combination reduces radical reactivity and therefore actin filament structure and VE-cadherin, as well as its barrier function, could be maintained. Cell morphology after given antioxidant combination showed cells membrane to be smooth and there are no damage cells. The combination of α–tocopherol and ascorbic acid has a beneficial effect, in which tocopheryl radical can be regenerated into tocopherol by ascorbic acid and therefore reduces stress oxidative [12, 22].
Based on this study, antioxidant combination needs a lower dose compared to single antioxidant to retain of endothelial barrier function. Ascorbic acid 50 µM and α–tocopherol 50 µM in single composition has not shown inhibition of permeability increase of HUVECS culture. Whereas, in the combination of ascorbic acid 50 µM and α–tocopherol 50 µM could reduce the increase of endothelial permeability until it was not different compared to the normal group. This combination also strongly decreased the endothelial permeability of the HUVEC culture.
The combination of ascorbic acid 100 µM and α–tocopherol 100 µM could prevent the permeability increase of HUVECS culture. However, it was lower compared to a dose each of 50 µM. The increase of dosage possibly changes it into a pro-oxidant effect due to the production increase of ascorbic and tocopheryl radical [11, 12]. Disorder of the transporter system of ascorbic acid has a potency to cause lipoprotein damage of cells membrane [6, 11]. They have a function as an endothelial barrier, therefore, the HUVEC’s permeability will increase. The metal transition also contributes to the increase of ROS production besides the expose of high concentration of glucose [2]. Ascorbic acid 25 µM and α–tocopherol 25 µM in combination did not significantly decrease endothelial permeability. This was caused by the low doses of its combination which cannot protect cells against oxidative damage, therefore, increase endothelial permeability [23]. This was signaled by the passage number of albumins which was high in the albuminal compartment.
Conclusion
Our studies showed that the exposure to high glucose concentration on HUVEC’s culture increases the endothelial permeability. Meanwhile, ascorbic acid 100 µM, α-tocopherol 100 µM, and 200 µM, as well as its combination potentially have protective properties by inhibiting the increase of endothelial cells permeability exposed a high concentration of glucose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding
This study was funded by Research Grant of Post Graduate from Directorate General of Higher Education Indonesia.
Conflict of interest
We declare that we have no conflict of interest.
- Pickup JC, Williams G, eds. Textbook of diabetes. 3rd edition. Oxford: Blackwell Science, 2003; 1520 p.
- Halliwel B, Gutteridge JMC. Free radical in biology and medicine. 5rd edition. Oxford: Oxford University Press, 2015; 896 p. https://doi.org/10.1093/acprof:oso/9780198717478.001.0001.
- Paolisso G, Giugliano D. Oxidative stress and insulin action: is there a relationship? Diabetologia 1996; 39(3): 357-363. https://doi.org/10.1007/BF00418354.
- Born GV, Shwartz CJ, eds. Vascular endothelium: physiology, pathology and therapeutic opportunities. Stuttgart: Schattauer, 1997; 391 p.
- Alexander JS. Rho, Tyrosine kinase, Ca2+, and junctions in endothelial hyperpermeability. Circ Res 2000; 87: 268-271. https://doi.org/10.1161/01.RES.87.4.268.
- Lum H, Roebuck KA. Oxidant Stress and Endothelial Cell Dysfunction. Am J Physiol Cell Physiol 2001; 280(4): C719-C741. http://doi.org/10.1152/ajpcell.2001.280.4.c719.
- Heiskala M, Peterson PA, Yang Y. The roles of claudin superfamily proteins in paracellular transport. Traffic 2001; 2(2): 93-98. https://doi.org/10.1034/j.1600-0854.2001.020203.x.
- Hempel A, Maasch C, Heintze U, Lindschau C, Dietz R, Luft FC, Haller H. High glucose concentrations increase endothelial cell permeability via activation of protein kinase Cα. Circ Res 1997: 81; 363-371. https://doi.org/10.1161/01.RES.81.3.363.
- Bonnefont-Rousselot D, Bastard JP, Jaudon MC, Delattre J. Consequences of the diabetic status on the oxidant/antioxidant balance. Diabetes Metab 2000; 26(3): 163-176. https://doi.org/10.1093/ndt/12.7.1399.
- Brigelius-Flohe R, Traber MG. Vitamin E: Function and metabolism. FASEB J 1999; 13(10): 1145-1155. http://doi.org/10.1096/fasebj.13.10.1145.
- Carr A, Frei B. Does vitamin C act as a pro oxidant under physiological condition? FASEB J 1999; 13(10): 1007-1024. http://doi.org/10.1096/fasebj.13.9.1007.
- Stahl W, Sies H. Antioxidant defense: vitamin C and E and carotenoids. Diabetes 1997; 46 Suppl 2: S14-S18. https://doi.org/10.2337/diab.46.2.S14.
- Guerci B, Böhme P, Kearney-Schwartz A, Zannad F, Drouin P. Endothelial dysfunction and type 2 diabetes. Part 2: altered endothelial function and the effects of treatments in type 2 diabetes mellitus. Diabetes Metab 2001; 27(4 Pt 1): 436-447. https://doi.org/10.4093/dmj.2013.37.5.393.
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007; 39(1): 44-84. https://doi.org/10.1016/j.biocel.2006.07.001.
- Dreher D, Junod AF. Differential effects of superoxide, hydrogen peroxide, and hydroxyl radical on intracellular calcium in human endothelial cells. J Cell Physiol 1995; 162(1): 147-153. https://doi.org/10.1002/jcp.1041620118.
- Kuchan MJ, Frangos JA. Shear stress regulates endothelin-1 release via protein kinase C and cGMP in cultured endothelial cells. Am J Physiol 1993; 264(1 Pt 2): H150-H156. http://doi.org/10.1152/ajpheart.1993.264.1.h150.
- Koya D, King GL. Protein Kinase C Activation and development of diabetic complication. Diabetes 1998; 47(6): 859-866. https://doi.org/10.2337/diabetes.47.6.859.
- Michel CC, Curry. Microvascular permeability. Physiol Rev 1999; 79(3): 704-745. https://doi.org/10.1152/physrev.1999.79.3.703.
- Schafer FQ, Wang HP, Kelley EE, Cueno KL, Martin SM, Buettner GR. Comparing β-carotene, vitamin E and nitric oxide as membrane antioxidants. Biol Chem 2002; 383(3-4): 671-681. http://doi.org/10.1515/bc.2002.069.
- Van Hinsbergh VM. Endothelial permeability for macromolecules. Arterioscler Thromb Vasc Biol 1997; 17(6): 1018-1023. https://doi.org/10.1161/01.ATV.17.6.1018.
- Pfaffly JR. Diabetes complication, hyperglycemia and free radical. Iowa: Bioscience Department University of Iowa, 2001; 18 p.
- Craven PA, DeRubertis FR, Kagan VE, Melhem M, Studer RK. Effects of supplementation with vitamin C or E on albuminuria, glomerular TGF-beta, and glomerular size in diabetes. J Am Soc Nephrol 1997; 8(9): 1405-1414. https://doi.org/10.2337/diabetes.50.9.2114.
- Brunton LL, Chabner BA, Knollmann BC. Goodman & Gilman's the pharmacological basis of therapeutics. New York: McGraw-Hill, 2011; 2084 p. https://www.moscmm.org/pdf/Goodman_and_Gilmans.pdf.
- Permatasari N. Changes in calcium cytosol level of human umbilical vein endothelial cells (HUVECs) culture exposed to high concentration of glucose and its relationship with H2O2 dan lipid peroxyde. PhD dissertation. Airlangga University, Surabaya, Indonesia, 2003. Malay. http://repository.unair.ac.id/32125/
- Nelwan CH. The role of Calcium cytosol level of Human Umbilical Vein Endothelial Cells (HUVECs) culture exposed to Glucose 22 mM on filamnent actin network. Thesis, Brawijaya University, Malang, Indonesia, 2004. Malay.
- Khotimah H. Effect vitamin C and vitamin E on activity of endothelial derived relaxing factor (EDRF) / nitric oxide (NO) and malondialdehyde (MDA) of human umbilical vein endothelial cells (HUVECs) culture exposed to supraphysiology level of glucose. Thesis, Brawijaya University, Malang, Indonesia, 2003. Malay.
Received 28 May 2018, Revised 16 August 2019, Accepted 18 February 2020
© 2018, Purnomo Y., Soeatmadji D.W, Widodo M.A.
© 2018, Russian Open Medical Journal
Correspondence to Yudi Purnomo. Address: Department of Pharmacology, Faculty of Medicine, Islamic University of Malang, Jl. Mayjen Haryono No. 193, Dinoyo, Lowokwaru, Malang, East Java 65144. Phone: (0341) 551932. E-mail: y_purnomo92@yahoo.com.