Introduction
Obesity is now one of the major problems of mankind. High obesity prevalence and considerable treatment cost of diseases associated with overweight determine the great social significance of this pathology. The overweight and obesity prevalence in the Russian Federation is 59.2% and 24.1%, respectively. According to the 7th UN report, in 2013 the Russian Federation ranked 19th among all countries in the world in terms of obesity prevalence. Results of the 2013 ESSE-RF multicenter study (Epidemiology of cardiovascular diseases and their risk factors in 11 regions of the Russian Federation) that included 25,224 individuals aged 25-64 years revealed that obesity prevalence in the population was 29.7% [1, 2].
It has been known from the earliest times that obesity is a disease. Hippocrates viewed excessive adipose tissue as a pathology and wrote the following: “Sudden death is more typical for obese than for lean people.” Cardiomyopathy in obese patients was for the first time described in detail in 1818 in the monograph by J. Cheyne “A case of apoplexy in which the fleshy part of the heart was converted into fat” [3]. Since then, many studies were performed that prove the negative effect of obesity on cardiovascular system. According to the latest data, abdominal fat is not only a lipid deposition but also a hormonally active tissue that produces dozens of biologically active substances: cytokines, adipokines and biologically active proteins that lead to cardiac remodeling [4]. Modern literary sources provide quite a few reviews of how obesity affects the risk of developing various cardiovascular diseases (CVD): arterial hypertension (AH), coronary heart disease (CHD), myocardial infarction (MI), various arrhythmias and chronic heart failures (CHF). Thus, a recently published meta-analysis demonstrated that obesity was significantly associated with an increased risk of CVD, including mortality. In patients with diabetes mellitus, AH or CHD, mortality had a U-shaped relation to their body mass index (BMI) [5]. A meta-analysis that included data from 21 studies (778,401 participants) focused on metabolic phenotypes. The MI risk was higher in metabolically unhealthy obese patients as compared to the patients with metabolically healthy normal weight phenotype, and the CHF risk was higher even in groups of patients with metabolically healthy overweight and metabolically healthy obesity. Moreover, healthy individuals with overweight and obesity demonstrated an increased risk of CVD. Thus, the researchers deduced that all metabolically unhealthy phenotypes in different weight categories were associated with an increased incidence of CVD, CHF and MI [6]. Another systematic review and meta-analysis of 29 studies describes the “J-curve” relation between BMI and possible HF development with the highest risk in patients with morbid obesity. The group of overweight patients demonstrated a lower cardiovascular mortality rate, which confirms the obesity paradox [7]. Another study of interest demonstrated that patients with BMI >45 kg/m2 were associated with higher prevalence of arterial hypertension, levels of systolic and diastolic blood pressure and C-reactive protein concentration, which means higher risk of CVD as compared to patients with BMI of 35.0-44.9 kg/m2 [8]. Obesity causes a number of social problems, such as retirement due to disability. The herein mentioned systematic review and meta-analysis of 25 prospective cohort studies that involved over two million participants demonstrated a J-curve relation between BMI and disability retirement: people with underweight, overweight and obesity were more likely to receive a disability pension for all the same reasons than people with normal weight. Herewith, overweight increased the risk of disability retirement due to musculoskeletal system disorders, while obesity, in addition to the above reason, also increased the risk of retirement due to cardiovascular diseases and mental disorders [9]. Obesity is undoubtedly a global public health problem, which is why such focused attention is being paid to this disease. Despite a great many studies in this area, the occurrence and progression pathogenesis of cardiac structural and functional changes in obese patients is still not fully understood. Most publications describe development of one or several CVDs only. The purpose of our review was to systematize the present-day knowledge, to describe in detail obesity impact on all aspects of a cardiovascular pathology development for a more in-depth understanding of the problem.
Myocardial remodeling
Obese patients demonstrate changes in the structure and morphology of the heart due to hemodynamic, metabolic and inflammatory shifts that are caused by excessive adipose tissue in the body. The most typical change is the development of left ventricular myocardial hypertrophy (LVH). LVH prevalence in patients with overweight is three times higher than in patients with normal body weight [10]. Genesis of such pathology is multifactorial.
First of all, we should mention leptin, a hormone produced by adipose tissue; obese patients demonstrate a significantly increased concentration of this hormone. It is known that increased concentration of leptin leads to a significantly increased risk of LVH. This is mainly due to arterial hypertension (AH), the most frequently associated pathology in obese patients that develops in case of hyperleptinemia. Leptin was proven to increase concentration of endogenous endothelin-1, a potent vasoconstrictor that possibly enhances activity of the sympathetic nervous system by increasing noradrenaline level in blood plasma, which leads to increased vascular tone and total peripheral vascular resistance. Also, leptin enhances platelet aggregation, stimulates production of reactive oxygen species, thereby provoking oxidative stress, which undoubtedly contributes to systemic inflammation and early progression of atherosclerosis that also increases the risk of AH [11]. Moreover, there is evidence that individuals with excessive adipose tissue develop LVH even in the absence of AH. A study by S. Adiarto et al. demonstrated increased expression of endothelin gene in cardiomyocytes and LVH development in response to increased leptin concentration. The authors advanced the hypothesis about independent impact of hyperleptinemia on myocardial hypertrophy development in the absence of FH and diabetes mellitus [12].
Another important hormone produced by adipose tissue is adiponectin. The data obtained revealed that the adiponectin level of under 10 μg/mL significantly increases the LV myocardium mass and LV myocardium mass index, which testifies to an inverse relationship between the thickness of the LV myocardium posterior wall and the adiponectin level. Administration of this hormone prevented LVH development [13]. It is argued that adiponectin inhibits development of myocardial hypertrophy caused by angiotensin II or AH through activation of the AMPK-dependent signaling pathway in cardiomyocytes [14]. Present-day literary sources include data on the ability of adiponectin in endothelial cells to enhance generation of nitric oxide and to suppress generation of reactive oxygen species [15, 16].
Another important adipocytokine that has an effect on LV myocardial hypertrophy is resistin. Resistin was proven to promote inflammation, insulin resistance, and atherosclerosis. Resistin causes endothelial dysfunction through production of adhesive molecules, endothelial migration, and smooth muscle cell proliferation. The effect of hyperresistinemia on myocardial remodeling in obese male adolescents has been described. Negative correlations between resistin and LV end-diastolic size and diastolic volume were revealed [17, 18].
It is also necessary to mention the lipotoxicity phenomenon that includes accumulation of plasma triglycerides in myocardium, which directly leads to gradual development of myocardial steatosis [19, 20].
It is not only hormonal and metabolic changes typical for obese patients that contribute to LVH development, but hemodynamic changes as well. Patients with overweight demonstrate increased circulating blood volume (CBV) and increased stroke volume. It is known that CBV is proportional to the volume of the vascular network of peripheral tissues. A rather intensive blood supply to adipose tissue significantly increases CBV in case of excessive adipose tissue [10]. Decreased peripheral vascular resistance that occurs in obese patients contributes to gradual deposition of blood leading to an even greater increase in CBV and stroke volume. Increased CBV in obese patients is also due to impaired renal function that leads to water and sodium salt retention. Chronic volume and pressure overload leads to formation of eccentric LVH, enlargement of heart chambers, cardiac remodeling and development of chronic heart failures (CHF) [4].
Obesity results not only in LV but also in RV hypertrophy. A number of studies demonstrated that RV size and thickness of its free wall are significantly larger in obese patients than in patients with normal weight [20]. RV hypertrophy found in obese patients is mainly a consequence of increased CBV, developed left ventricular failure and respiratory failure during sleep [15]. To a certain extent, pulmonary hypertension progressing due to hypoxia caused by sleep apnea syndrome also contributes to the development of right ventricular failure [10].
Development of arrhythmias and conduction abnormalities
In recent years, it has become undeniable that obesity is a predictor of arrhythmia development. Thus, according to the results of the Atherosclerosis Risk in Communities Study, it is obesity that determines the risk of arrhythmia in 17.9% cases [21]. The most common and clinically significant rhythm disturbance is atrial fibrillation (AF). Its prevalence is constantly increasing, its incidence in the general population reaches 2%. AF worsens the patients’ quality of life and increases the risk of fatal cardiovascular complications [22, 23]. Steadily increasing incidence of AF is largely due to the increase of obesity in the population. C. Wong et al. demonstrated an increase in AF risk by 3.5-5.3% for each unit of the body mass index (BMI) [24]. The Framingham Heart Study revealed that every fifth patient with AF is obese [25]. Other studies showed relationship between obesity and arrhythmia development was found even in young and middle-aged patients with no history of cardiovascular diseases [26]. Based on the results obtained, special calculators were developed to assess the risk of AF development [27]. A study by R. Sandhu et al. showed that obesity was associated mostly with the development of persistent AF [28].
Obesity is currently viewed as a major factor that increases the AF risk due to activation of the sympathoadrenal nervous system, increased activity of the renin-angiotensin-aldosterone system, development of insulin resistance, lipid metabolism disorders, and more frequent AH development in such patients [22, 29].
It is known that enlargement of the left atrial cavity (LA) is the main risk factor of AF development. The Framingham Heart Study showed that LA size was directly proportional to the risk of such arrhythmia development [30]. As of today, there is a lot of published data on increased LA in obese patients [31]. Basic mechanisms provoking LA increase in patients with excessive adipose tissue are as follows: volume overload due to a significant increase in CBV, AH and LV diastolic dysfunction that is often found in obese patients [4].
Lipid storage disorders also contribute to the risk of AF. Increased level of triglycerides, low-density lipoprotein cholesterol, and decreased level of high-density lipoprotein cholesterol are found in obese patients more often than in the general population. High concentration of triglycerides contributes to increased free fatty acids in blood plasma. The study by Khawaja O. et al. demonstrated arrhythmogenic effects of free fatty acids. Possible pathogenetic mechanisms of AF development in this case are as follows: decreased glucose oxidation and accumulation of acidic metabolic products in cardiomyocytes, activation of lipid peroxidation, release of lysophospholipids from damaged membranes of cardiomyocytes [32, 33].
Progressive obesity leads to deposition of fat not only in typical fat depots (abdominal fat) but also around various organs, including the heart. Accumulation of visceral fat between epicardium and the visceral layer of pericardium is called epicardial adipose tissue (EAT). The study by R. Mahajan demonstrated that obesity is accompanied by a significant increase in its volume [34]. Negative effect of EAT is multifactorial: there is a mechanical effect and pressure on the heart; there is a gradual accumulation of fatty deposits between muscle fibers and partial replacement of myocytes with adipocytes that secrete biologically active substances with direct and indirect cardiotoxic effects. Lipotoxic effect of adipose tissue is due to the fact that lipids are accumulated not only in adipocytes but also in other cells of the body including myocytes and endotheliocytes, and cause their dysfunction and apoptosis. Therefore, progression of steatosis inevitably leads to fibrosis development that is the main risk factor of cardiac arrhythmia development. Fat accumulations in the heart are in the form of stripes and originate from epicardial fat; they are largely accumulated in the region of the right ventricle and atrioventricular groove. Thus, the process of fatty infiltration of the heart muscle involves, first of all, the right ventricle, followed by sinus and atrioventricular nodes [4]. The Framingham Heart Study revealed that increased EAT was accompanied by increased LA and contributed to the development and long-term persistence of AF [22, 35]. Negative effect of EAT on the heart is due to the high hormonal activity of this tissue. It was proven that EAT macrophages secrete a large amount of proinflammatory cytokines that have a local arrhythmogenic effect on atrial myocardium. The level of expression of proinflammatory cytokines in EAT is much higher than in visceral adipose tissue in other localizations. EAT macrophages synthesize C-reactive protein, myeloperoxidase, interleukins, tumor necrosis factor-alpha, transforming growth factor, heat shock proteins, galectin-3 [22, 32, 36]. Leptin plays a significant role in the development of AF; its concentration in obese patients is significantly higher. A number of studies demonstrated accelerated development of atrial fibrosis associated with excessive concentration of leptin that significantly increased the possibility of AF development [37].
EAT is anatomically associated with myocardium; infiltration of myocardium by dysfunctional adipose tissue accompanied by inflammation and fibrosis processes contributes to electrical remodeling of atria that leads to impaired intra-atrial conduction [38, 39]. The study by Friedman D. et al. showed the relationship between EAT and slowing of atrial impulse conduction time; it was suggested that pericardial fat alters the electrophysiology of atria and increases the risk of arrhythmias [40]. Modern literary sources show that infiltration of cardiac conducting structures (sinus node, atrioventricular node, bundle branch) with fat cells can be accompanied by conduction disorders along these pathways; there are also reports of more frequent development of supraventricular and ventricular extrasystole, supraventricular tachycardia in patients with excessive adipose tissue [41, 42]. The study performed by A. Zemva showed that obese people have an increased frequency of ventricular extrasystoles as compared to patients with normal body weight, and this is not associated with AH or ventricular hypertrophy [43]. E.I. Panova et al. analyzed the characteristics of the heart rhythm in men of working age with AH and obesity and concluded that obesity in combination with AH increases the incidence of AF by 15.3%, and of ventricular premature beats – by 26.5%, as compared to people with normal body weight, while there was also a connection between arrhythmias and the severity of obesity. The relative risk of AF development in patients with AH combined with obesity increased by 6.2 times, and the risk of ventricular premature beats – by 5.31 times. Additional risk factors of ventricular premature beats included CHD, angina, LV hypertrophy, and LA dilatation. This paper also shows the relationship between the number of ventricular extrasystoles per day and the adiponectin level [44]. Obese patients are characterized by tachycardia caused by activation of the sympathoadrenal nervous system. Mechanisms of proarrhythmic electrophysiological remodeling in obese patients are not clearly understood. Obese patients demonstrate prolonged QT interval, increased frequency of ventricular extrasystoles, and increased risk of sudden cardiac death that is usually arrhythmogenic [45]. Analysis of the electrophysiological state of myocardium in patients with obesity and metabolic syndrome revealed sinus node dysfunction, lengthening of refractory periods of α and β pathways of AV junction [44, 46].
Diastolic dysfunction
In recent years, clinicians and physiologists focused on the study of development mechanisms of myocardium diastolic dysfunction (DD) and its role in the onset of CHF. Numerous studies demonstrated that DD can serve as a reliable and sensitive marker of both early and later myocardial damage. Disorders of the active relaxation of ventricular myocardium and impaired compliance of ventricular walls that underlie DD are most often found in case of atherosclerosis, myocardial ischemia, AH, and diabetes mellitus [47]. The impact of obesity on DD development is actively discussed in modern literary sources. A number of research papers showed that asymptomatic impairment of LV diastolic function developed in patients with abdominal obesity (AO) even before the onset of clinical signs of a heart failure [48, 49]. The results of the study by E.V. Bazhenova et al. prove the effect of obesity on the development and further progression of LV DD. It was found that the risk of LV DD development in obese patients without concomitant AH with a BMI of ≥30.0 kg/m2 increases by 3.7 times [50]. Analysis of the results of the study by I.R. Popova reveals that the prevalence of LV DD in obese patients is 2 times higher than in people with normal BMI. A direct correlation was found between LV DD and BMI [10]. The study by Tsujimoto T. et al. showed that obesity was one of the early predictors of increased mortality in patients with a heart failure with preserved LV ejection fraction (EF) [51]. AO plays a special role in the development and progression of DD. Thus, C. Russo et al. demonstrated that AO can be a risk factor of LV dysfunction development regardless of the presence of general obesity [52].
Pathogenesis of LV DD development in obese patients still remains a poorly understood issue. Most scientists believe that DD in obese patients is caused by combined influence of metabolic and hemodynamic factors associated with excessive abdominal and visceral adipose tissue [50, 53, 54]. Obesity is associated with structural and functional changes in LV that lead to LV hypertrophy and increased cardiac output. Insulin resistance and increased oxidative stress observed in obese patients exacerbate myocardial relaxation disorders and provoke early development of DD [52]. The study by Rayner J. demonstrated the negative effect of visceral fat on diastolic function due to concentric remodeling of LV myocardium, increased triglyceride levels in myocardium, and impaired myocardial metabolism. According to their data, it is the change in myocardial metabolism that has the most effect on the formation of DD in obese patients [48]. Thus, myocardial steatosis and lipotoxicity make a significant contribution to the development of this pathology [55]. The research paper by Gritsenko O.V. et al. demonstrated the relationship between EAT thickness and the levels of pro-inflammatory cytokines (tumor necrosis factor-α, interleukin-6), adipokines (leptin and adiponectin) and markers of myocardial fibrosis (matrix metalloproteinase-3, transforming growth factor-β and collagen). Based on the results of their work, it can be concluded that elevated profibrotic factors in patients with increased EFT thickness of ≥7 mm may be a marker of preclinical and, therefore, earlier signs of lipotoxic myocardial fibrosis that was not detected by echocardiographic examination in the form of DD [56].
Systolic dysfunction
The main problem with assessing obesity impact on LV systolic function is that patients with excessive adipose tissue and decreased LVEF, normally, already demonstrate a combined pathology, such as CHD, AH, diabetes mellitus, which are indisputable causes of LV systolic dysfunction (SD). Therefore, it is very difficult to study pathogenetic mechanisms of SD development with underlying obesity. The results of studies of LV systolic function in obese patients are rather contradictory. Some authors report decreased LVEF, others report normal or even increased LVEF (so-called hyperdynamic heart response with an increase in EF of over 80%) in obese patients. It should be noted that the authors of such articles always mention that it is difficult to separate the negative impact of obesity from the consequences associated with the diseases of cardiovascular system [53]. Such inconsistency in the research results may, among other things, be explained by a reflex increase in endocardial excursion surrounded by a hypertrophied layer of myocardium in the early stages of LV remodeling in case of obesity. Applying Simpson’s technique that includes measuring the rate of systolic expulsion of blood from LV made it possible to find that patients with initial stages of obesity demonstrated decreased myocardial contractility even with normal LVEF [4, 31]. A number of studies of obese children and adolescents who were diagnosed with LVH with impaired systolic function showed the negative effect of obesity on the development of DM and LV systolic dysfunction [57]; similar changes were registered in obese patients at the age of thirty, forty and fifty years, which negates the correlation between an impaired LV systolic function and the patient’s age. Animal studies revealed that impaired LV systolic function in case of obesity was due to a decreased myocardial sensitivity to calcium ions and decreased number of β-receptors in the heart muscle [4].
Questions about whether relatively mild changes in myocardial systolic function progress with a longer period of obesity and whether prolonged obesity leads to heart failures regardless of CHD or other diseases remain open. Alpert et al. suppose that the duration of obesity is a factor determining the possibility of development of DM, LV systolic dysfunction, and heart failures [58].
Pathogenesis of CHF in obese patients is based on the deterioration of myocardial compensatory mechanisms due to increased hemodynamic load. It was proven that cardiac output and stroke volume are significantly increased in obese patients, even in the absence of diabetes mellitus, AH, and other heart diseases, and they reliably correlate with the patients’ weight [59]. Increased cardiac output in obese patients is due to the need to meet the increased metabolic requirements of excessive adipose tissue. As the amount of adipose tissue increases, resting heart rate increases, which is caused by activation of the sympathetic nervous system and decreased activity of the parasympathetic nervous system. Tachycardia further increases cardiac output. Increased stroke volume and cardiac output are also the result of gradually increased CBV, which is a compensatory reaction in response to the enlarged volume of vascular bed associated with increased body weight. Increased CBV and cardiac output lead to decreased peripheral resistance. Thus, the basic pathogenetic mechanism for CHF development in obese patients is the deterioration of myocardial compensatory capabilities due to increased CBV being an adaptive response to accumulation of excessive adipose tissue [60].
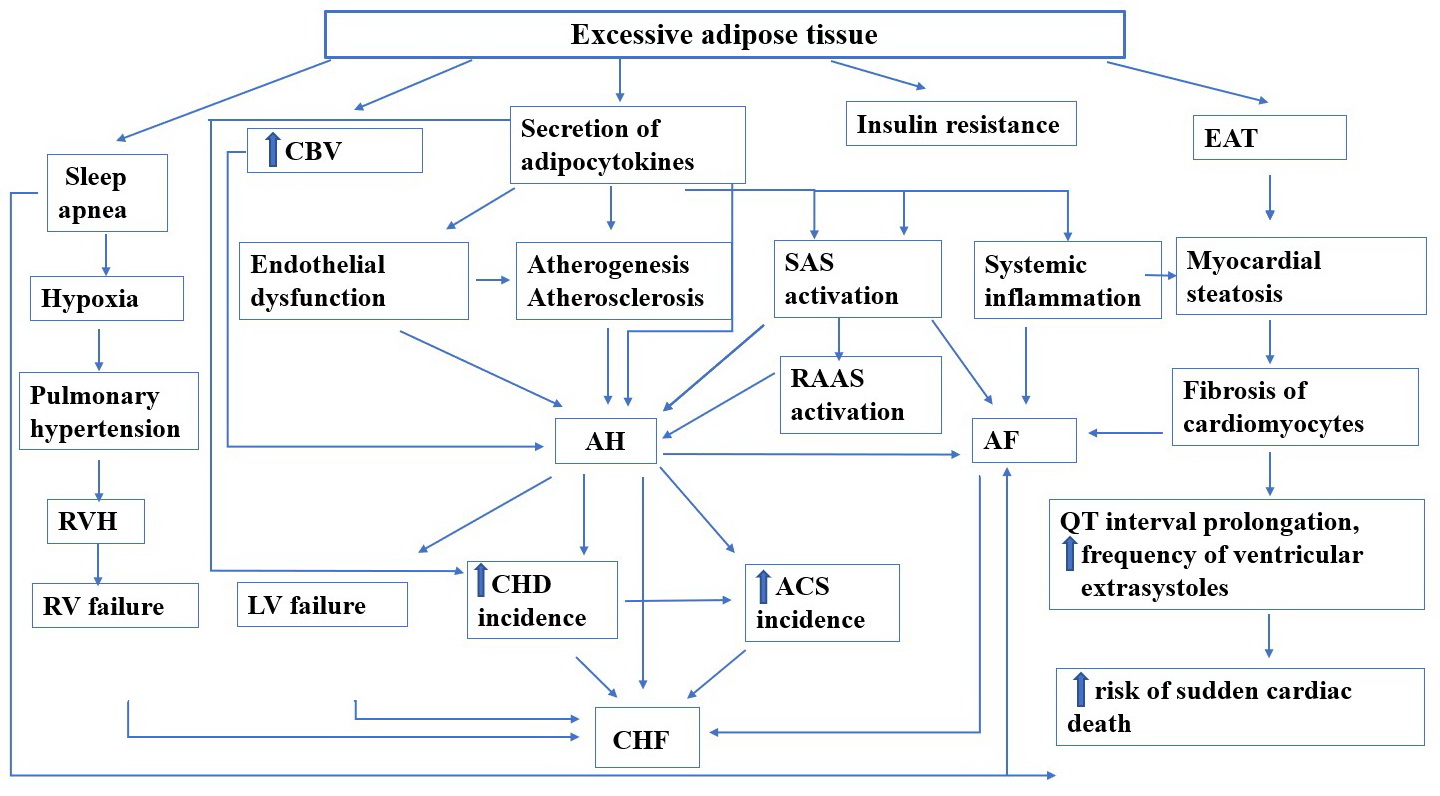
Figure 1 shows the impact of excessive adipose tissue.
Figure 1. Obesity impact on cardiovascular system.
CBV, circulating blood volume; EAT, epicardial adipose tissue; SAS, sympathoadrenal system; RAAS, renin-angiotensin-aldosterone system; AH, arterial hypertension; AF, atrial fibrillation; RV hypertrophy, right ventricular hypertrophy; CHD, coronary heart disease; ACS, acute coronary syndrome; CHF, chronic heart failure.
Conclusion
The obesity problem is currently a priority medical challenge. The modern literature views obesity not only as an undeniable risk factor of cardiovascular diseases but also as a disease that increases the risk of a sudden cardiac death [41, 45]. The scientific novelty of this article consists in a detailed and unified presentation of such data. There are many publications that describe the negative impact of obesity on the development of cardiac structural and functional changes that lead to left ventricular hypertrophy, rhythm and conduction abnormalities, development and progression of chronic heart failures. Physicians require understanding of pathogenetic aspects of obesity impact on CVD development for a personalized approach to patient treatment, which is undoubtedly the main trend in modern medicine. Measures aimed at promotion of a healthy lifestyle and proper nutrition of the population and timely treatment of obesity will undoubtedly help reduce cardiovascular morbidity and improve the quality of life and life expectancy.
Conflict of interest
All authors declare that there is no potential conflict of interest.
- Diagnosis, treatment, prevention of obesity and associated diseases. National clinical guidelines. St. Petersburg. 2017; 164 p. Russian. https://scardio.ru/content/Guidelines/project/Ozhirenie_klin_rek_proekt.pdf.
- Muromtseva GA, Kontsevaya AV, Konstantinov VV, Artamonova GV, Gatagonova TM, Duplyakov DV, et al. The prevalence of non-infectious diseases risk factors in Russian population in 2012-2013 years. The results of ECVD-RF. Cardiovascular Therapy and Prevention 2014; 13(6): 4-11. Russian. http://doi.org/10.15829/1728-8800-2014-6-4-11.
- Fletcher A, Moor D. The lives and works of John Cheyne (1777-1836) and William Stokes (1804-1878). J Intensive Care Soc 2017; 18(4): 323-325. https://doi.org/10.1177/1751143717702929.
- Chumakova GA, Veselovskaya NG, Kozarenko AA, Vorobyeva YuV. Heart morphology, structure, and function in obesity. Russian Journal of Cardiology 2012; 17(4): 93-99. Russian. https://www.elibrary.ru/item.asp?id=17904015.
- Dwivedi AK, Dubey P, Cistola DP, Reddy SY. Association Between Obesity and Cardiovascular Outcomes: Updated Evidence from Meta-analysis Studies. Curr Cardiol Rep 2020; 22(4): 25. https://doi.org/10.1007/s11886-020-1273-y.
- Mirzababaei A, Djafarian K, Mozafari H, Shab-Bidar S. The long-term prognosis of heart diseases for different metabolic phenotypes: a systematic review and meta-analysis of prospective cohort studies. Endocrine 2019; 63(3): 439-462. https://doi.org/10.1007/s12020-019-01840-0.
- Mahajan R, Stokes M, Elliott A, Munawar DA, Khokhar KB, Thiyagarajah A, et al. Complex interaction of obesity, intentional weight loss and heart failure: a systematic review and meta-analysis. Heart 2020; 106(1): 58-68. https://doi.org/10.1136/heartjnl-2019-314770.
- Santos ASAC, Rodrigues APS, Rosa LPS, Sarrafzadegan N, Silveira EA. Cardiometabolic risk factors and Framingham Risk Score in severely obese patients: Baseline data from DieTBra trial. Nutr Metab Cardiovasc Dis 2020; 30(3): 474-482. https://doi.org/10.1016/j.numecd.2019.10.010.
- Shiri R, Falah-Hassani K, Lallukka T. Body mass index and the risk of disability retirement: a systematic review and meta-analysis. Occup Environ Med 2020; 77(1): 48-55. https://doi.org/10.1136/oemed-2019-105876.
- Popova IR, Drapkina OM. Obesity and Chronic Heart Failure. Lechebnoye delo 2012; (3): 68-73. Russian. https://www.elibrary.ru/item.asp?id=22923415.
- Correia MLG, Haynes WG. Leptin, obesity and cardiovascular disease. Curr Opin Nephrol Hypertens 2004; 13(2): 215-223. https://doi.org/10.1097/00041552-200403000-00010.
- Adiarto S, Emoto N, Iwasa N, Yokoyama M. Obesity-induced upregulation of myocardial endothelin-1 expression is mediated by leptin. Biochem Biophys Res Commun 2007; 353(3): 623-627. https://doi.org/10.1016/j.bbrc.2006.12.066.
- Verbovoy AF, Mitroshina EV. Adipokines and cardiovascular system. Endocrinology: News, Opinions, Training 2014; (2(7)): 11-17. Russian. https://www.elibrary.ru/item.asp?id=22779756.
- Karpushev AV, Mikhailova VB. The role of adipokines in the regulation of cardiovascular function. Arterial Hypertension 2019; 25(5): 448-459. Russian. https://doi.org/10.18705/1607-419X-2019-25-5-448-459.
- Alpert MA, Karthikeyan K, Abdullah O, Ghadban R. Obesity and Cardiac Remodeling in Adults: Mechanisms and Clinical Implications. Prog Cardiovasc Dis 2018; 61(2): 114-123. https://doi.org/10.1016/j.pcad.2018.07.012.
- Zhou Y, Wei Y, Wang L, Wang X, Du X, Sun Z, et al. Decreased adiponectin and increased inflammation expression in epicardial adipose tissue in coronary artery disease. Cardiovasc Diabetol 2011; 10: 2. https://doi.org/10.1186/1475-2840-10-2.
- Verbovoy AF, Tsanava IA, Verbovaya NI, Galkin RA. Resistin – a marker of cardiovascular diseases. Obesity and Metabolism 2017; 14(4): 5-9. Russian. https://doi.org/10.14341/omet201745-9.
- Unger RH. Lipotoxic Diseases. Annu Rev Med 2002; 53: 319-336. https://doi.org/10.1146/annurev.med.53.082901.104057.
- Unger RH, Scherer PE. Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity. Trends Endocrinol Metab 2010; 21(6): 345-352. https://doi.org/10.1016/j.tem.2010.01.009.
- Wong CY, O’Moore-Sullivan T, Leano R, Hukins C, Jenkins C, Marwick TH. Association of subclinical right ventricular dysfunction with obesity. J Am Coll Cardiol 2006; 47(3): 611-616. https://doi.org/10.1016/j.jacc.2005.11.015.
- Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the atherosclerosis risk in communities (ARIC) study. Circulation 2011; 123(14): 1501-1508. https://doi.org/10.1161/circulationaha.110.009035.
- Podzolkov VI, Tarzimanova AI, Gataulin RG, Oganesyan KA, Lobova NV. The role of obesity in the development of atrial fibrillation: current problem status. Cardiovascular Therapy and Prevention 2019; 18(4): 109-114. Russian. https://doi.org/10.15829/1728-8800-2019-4-109-114.
- Krijthe BP, Kunst A, Benjamin EJ, Lip GY, Franco OH, Hofman A, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J 2013; 34(35): 2746-2751. https://doi.org/10.1093/eurheartj/eht280.
- Wong CX, Sullivan T, Sun MT, Mahajan R, Pathak RK, Middeldorp M, et al. Obesity and the risk of incident, post-operative, and post-ablation atrial fibrillation: a meta-analysis of 626,603 individuals in 51 studies. JACC Clin Electrophysiol 2015; 1(3): 139-152. https://doi.org/10.1016/j.jacep.2015.04.004.
- Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, et al. 50-year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet 2015; 386(9989): 154-162. https://doi.org/10.1016/s0140-6736(14)61774-8.
- Karasoy D, Bo Jensen T, Hansen ML, Schmiegelow M, Lamberts M, Gislason GH, et al. Obesity is a risk factor for atrial fibrillation among fertile young women: a nationwide cohort study. Europace 2013; 15(6): 781-786. https://doi.org/10.1093/europace/eus422.
- Chamberlain AM, Agarwal SK, Folsom AR, Soliman EZ, Chambless LE, Crow R, et al. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] study). Am J Cardiol 2011; 107(1): 85-91. https://doi.org/10.1016/j.amjcard.2010.08.049.
- Sandhu RK, Conen D, Tedrow UB, Fitzgerald KC, Pradhan AD, Ridker PM, et al. Predisposing factors associated with development of persistent compared with paroxysmal atrial fibrillation. J Am Heart Assoc 2014; 3(3): e000916. https://doi.org/10.1161/JAHA.114.000916.
- Goudis CA, Korantzopoulos P, Ntalas IV, Kallergis EM, Ketikoglou DG. Obesity and atrial fibrillation: A comprehensive review of the pathophysiological mechanisms and links. J Cardiol 2015; 66(5): 361-369. https://doi.org/10.1016/j.jjcc.2015.04.002.
- Wang TJ, Parise H, Levy D, D'Agostino RB, Wolf PA, Vasan RS, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA 2004; 292(20): 2471-2477. https://doi.org/10.1001/jama.292.20.2471.
- Avelar E, Cloward TV, Walker JM, Farney RJ, Strong M, Pendleton RC, et al. Left ventricular hypertrophy in severe obesity: interactions among blood pressure, nocturnal hypoxemia, body mass. Hypertension 2007; 49(1): 34-39. https://doi.org/10.1161/01.hyp.0000251711.92482.14.
- Drapkina ОМ, Nikolaeva МV. Pathogenic Mechanisms of Atrial Fibrillation in Obesity. Rational Pharmacotherapy in Cardiology 2016; 12(5): 582-589. Russian. https://doi.org/10.20996/1819-6446-2016-12-5-582-589.
- Khawaja O, Bartz TM, Ix JH, Heckbert SR, Kizer JR, Zieman SJ, et al. Plasma free fatty acids and risk of atrial fibrillation (from the Cardiovascular Health Study). Am J Cardiol 2012; 110(2): 212-216. https://doi.org/10.1016/j.amjcard.2012.03.010.
- Mahajan R, Lau DH, Brooks AG, Shipp NJ, Manavis J, Wood JP, et al. Electrophysiological, electroanatomical, and structural remodeling of the atria as consequences of sustained obesity. J Am Coll Cardiol 2015; 66(1): 1-11. https://doi.org/10.1016/j.jacc.2015.04.058.
- Fox CS, Gona P, Hoffmann U, Porter SA, Salton CJ, Massaro JM, et al. Pericardial fat, intrathoracic fat, and measures of left ventricular structure and function: the Framingham Heart Study. Circulation 2009; 119(12): 1586-1591. https://doi.org/10.1161/circulationaha.108.828970.
- Kondo H, Abe I, Gotoh K, Fukui A, Takanari H, Ishii Y, et al. Interleukin 10 Treatment Ameliorates High-Fat Diet-Induced Inflammatory Atrial Remodeling and Fibrillation. Circ Arrhythm Electrophysiol 2018; 11(5): e006040. https://doi.org/10.1161/circep.117.006040.
- Scridon A, Dobreanu D, Chevalier P, Şerban RC. Inflammation, a link between obesity and atrial fibrillation. Inflamm Res 2015; 64(6): 383-393. https://doi.org/10.1007/s00011-015-0827-8.
- Lin YK, Chen YJ, Chen SA. Potential atrial arrhythmogenicity of adipocytes: Implications for the genesis of atrial fibrillation. Med Hypotheses 2010; 74(6): 1026-1029. https://doi.org/10.1016/j.mehy.2010.01.004.
- Druzhilov MA, Kuznetsova TYu. Obesity associated atrial fibrillation: epicardial fat tissue in etiopathogenesis. Russian Journal of Cardiology 2017; 22(7): 178-184. Russian. http://doi.org/10.15829/1560-4071-2017-7-178-184.
- Friedman DJ, Wang N, Meigs JB, Hoffmann U, Massaro JM, Fox CS, et al. Pericardial fat is associated with atrial conduction: the Framingham Heart Study. J Am Heart Assoc 2014; 3(2): e000477. https://doi.org/10.1161/jaha.113.000477.
- Pathak RK, Mahajan R, Lau DH, Sanders P. The Implications of Obesity for Cardiac Arrhythmia Mechanisms and Management. Can J Cardiol 2015; 31(2): 203–210. https://doi.org/10.1016/j.cjca.2014.10.027.
- Provotorov VM, Glukhovsky ML. Rhythm and conductivity disorders in patients at the initial stages of metabolic syndrome. Clinical Medicine 2009; 87(7): 26-28. Russian. https://www.elibrary.ru/item.asp?id=12805824.
- Zemva A, Zemva Z. Ventricular ectopic activity, left ventricular mass, hyperinsulinemia, and intracellular magnesium in normotensive patients with obesity. Angiology 2000; 51(2): 101-106. https://doi.org/10.1177/000331970005100202.
- Tsyplenkova NS, Panova EI. Heart rate features among men of working age with obesity and hypertension. Obesity and metabolism 2016; 13(1): 30-35. Russian. https://doi.org/10.14341/omet2016130-35.
- Huang H, Amin V, Gurin M, Wan E, Thorp E, Homma S, et al. Diet-induced obesity causes long QT and reduces transcription of voltage-gated potassium channels. J Mol Cell Cardiol 2013; 59: 151-158. https://doi.org/10.1016/j.yjmcc.2013.03.007.
- Iskenderov BG, Lokhina TV, Shibaeva ТМ, Kapelovich VYu, Kazantseva LV. Dynamics of electrophysiological parameters of the heart in hypertensive patients depending on a 24-h profile of arterial pressure, left ventricular geometry and metabolic disorders. Therapeutic Archive 2006; 78(9): 12-17. Russian. https://www.elibrary.ru/item.asp?id=9293959.
- Mrikaev DV. Left ventricular diastolic dysfunction in patients with heart failure. Creative Сardiology 2017; 11(2): 145-158. Russian. https://doi.org/10.24022/1997-3187-2017-11-2-145-158.
- Rayner JJ, Banerjee R, Holloway CJ, Lewis AJM, Peterzan MA, Francis JM, et al. The relative contribution of metabolic and structural abnormalities to diastolic dysfunction in obesity. Int J Obes (Lond) 2018; 42(3): 441–447. https://doi.org/10.1038/ijo.2017.239.
- Selvaraj S, Martinez EE, Aguilar FG, Kim KY, Peng J, Sha J, et al. Association of central adiposity with adverse cardiac mechanics: findings from the hypertension genetic epidemiology network study. Circ Cardiovasc Imaging 2016; 9(6): e004396. https://doi.org/10.1161/circimaging.115.004396.
- Bazhenova EA, Karonova TL, Nikolaychuk EI, Lozovaya TA, Nifontov SE, Listopad OV, et al. Disorders of left ventricular diastolic function and anthropometric parameters in patients with abdominal obesity. Arterial Hypertension 2018; 24(1): 65-73. Russian. https://doi.org/10.18705/1607-419X-2018-24-1-65-73
- Tsujimoto T, Kajio H. Abdominal obesity is associated with an increased risk of all-cause mortality in patients with HFpEF. J Am Coll Cardiol 2017; 70(22): 2739-2749. https://doi.org/10.1016/j.jacc.2017.09.1111.
- Russo C, Sera F, Jin Z, Palmieri V, Homma S, Rundek T, et al. Abdominal adiposity, general obesity, and subclinical systolic dysfunction in the elderly: A population-based cohort study. Eur J Heart Fail 2016; 18(5): 537-544. https://doi.org/10.1002/ejhf.521.
- Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev 2008; 88(2): 389-419. https://doi.org/10.1152/physrev.00017.2007.
- Dutheil F, Gordon BA, Naughton G, Crendal E, Courteix D, Chaplais E, et al. Cardiovascular risk of adipokines: a review. J Int Med Res 2018; 46(6): 2082-2095. https://doi.org/10.1177/0300060517706578.
- Banerjee R, Rial B, Holloway CJ, Lewandowski AJ, Robson MD, Osuchukwu C, et al. Evidence of a direct effect of myocardial steatosis on LV hypertrophy and diastolic dysfunction in adult and adolescent obesity. JACC Cardiovasc Imaging 2015; 8(12): 1468-1470. https://doi.org/10.1016/j.jcmg.2014.12.019.
- Gritsenko OV, Chumakova GA, Gruzdeva OV, Shevlyakov IV. The relationship of epicardial obesity and levels of cardiac fibrosis markers. Russian Journal of Cardiology 2019; 24(4): 13-19. Russian. https://doi.org/10.15829/1560-4071-2019-4-13-19.
- Chinali M, de Simone G, Roman MJ, Lee ET, Best LG, Howard BV, et al. Impact of obesity on cardiac geometry and function in a population of adolescents: the Strong Heart Study. J Am Coll Cardiol 2006; 47(11): 2267-2273. https://doi.org/10.1016/j.jacc.2006.03.004.
- Alpert MA, Lambert CR, Panayiotou H, Terry BE, Cohen MV, Massey CV, et al. Relation of duration of morbid obesity to left ventricular mass, systolic function, and diastolic filling, and effect of weight loss. Am J Cardiol 1995; 76(16): 1194-1197. https://doi.org/10.1016/s0002-9149(99)80338-5.
- Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol 2009; 53(21): 1925-1932. https://doi.org/10.1016/j.jacc.2008.12.068.
- Gritsenko ОV, Chumakova GА, Shevlyakov IV, Trubina ЕV. The mechanisms of heart failure development in obesity. Russian Journal of Cardiology 2018; 23(5): 81-86. Russian. http://doi.org/10.15829/1560-4071-2018-5-81-86.
Received 19 October 2020, Revised 2 August 2021, Accepted 3 September 2021
© 2020, Russian Open Medical Journal
Correspondence to Anna E. Pokrovskaya. E-mail: a.e.pokrovskaya@mail.ru.