Introduction
Recently, the need for safe and high-quality blood exhibited and increasing trend with technological advances in the healthcare sector, especially in the case of blood management in Indonesia [1]. Packed red blood cells (PRC) are most commonly used. Currently, leukoreduced PRC (with removed white blood cells) have become widely used. Leukoreduced PRC are often used for repeated transfusions because they reduce human leukocyte antigen alloimmunization and post-transfusion reactions, such as febrile non-hemolytic transfusion reactions [2, 3].
Erythrocytes in the PRC function as oxygen transporters; hence, they must have good membrane resistance when flowing through a capillary with a smaller diameter so that they can circulate in the tissues. The resistance of the erythrocyte membrane depends on changes in its composition and structure, age of erythrocytes and the presence of sodium (Na), potassium (K) and calcium (Ca) cations. In addition, the pumping of ions helps maintaining the fluidity and permeability of erythrocyte membranes. The balance of these ions inside the cell can control the deformability and structure of erythrocytes, making them flexible [4].
Moreover, the permeability of the red blood cell membrane can modify through biochemical, physiological, or morphological processes during a specific period in the course of PRC storage, commonly called a storage lesion [2]. The storage lesion can generate a reduction in ATP concentration and 2,3- diphosphoglycerate (2,3-DPG); fatigue of the endogenous antioxidant defense system due to oxidative stress, hemoglobin (Hb) autoxidation, and leakage of lactate dehydrogenase; and potassium and calcium ions in packed blood, which affects red blood cell membrane resistance [2, 5].
In vitro PRC are different from blood in the human body, which causes a decrease in viability due to storage lesion. To maintain viability in the PRC, the anticoagulant agent, citrate phosphate dextrose adenine (CPDA-1), is added to extend the life of red blood cells up to 35 days in the blood bank at a storage temperature of 2–6 ℃ [6]. Leukoreduced PRC attempts improving its viability: white blood cells secrete cytokine, interleukin, protease and hydrogen peroxide. Hydrogen peroxide can cause structural changes in the membrane and increase its rigidity, affecting the resistance of cell membrane and causing hemolysis [7].
In addition, an increase in white blood cells can be caused by chemicals found in cigarettes. This may promote the release of proinflammatory cytokines, forming free radicals in the human body [8]. In fact, cigarettes contain over 7,000 chemicals, 250 of which threaten health. These chemicals are carcinogenic and mutagenic and contain heavy metals and free radicals that can generate reactive oxygen species (ROS) and reactive nitrogen species [9].
The World Health Organization (WHO) uses the term active smokers to refer to active smoking on a daily basis for six months throughout life. Besides, WHO classifies smokers into three groups based on the daily number of smoked cigarettes: light smokers (1-10), moderate smokers (11-20), and heavy smokers (>20 cigarettes) [10]. In the erythrocytes of smokers, oxidant-induced hemolysis modifies deformability, osmotic fragility, and susceptibility, which leads to oxidative stress [11].
Oxidative stress is when antioxidants become unable to fight the free radicals. Generated in the body of a smoker, they lead to aging of red blood cells and damage to their membranes, causing oxidative stress. Human body is naturally equipped with a protective mechanism of antioxidants against free radicals [12]. In addition, some antioxidants in the erythrocytes of smokers, such as glutathione peroxidase, catalase, and superoxide dismutase (SOD), tend to decrease [13]. For example, the enzyme SOD in smokers is relatively lower than in non-smokers due to the formation of free radicals, especially hydrogen peroxide, formed by direct interaction of cigarette smoke with tissues and causing a decrease in SOD [14, 15]. Moreover, the reduction of SOD enzyme in erythrocytes causes extensive production of antioxidants, which can damage the lipid membrane of erythrocytes and increase lipid peroxidation [16].
Sustained lipid peroxidation can break the chain of polyunsaturated fatty acids (PUFAs) into some toxic compounds, viz. aldehydes, such as malondialdehyde (MDA) [16]. Furthermore, the duration of smoking can affect peroxidation of lipids, which causes an increase in MDA. Norah et al. found a strong correlation between increasing MDA and smoking duration [14]. An increase in the concentration of MDA indicates a progressive damage to the membrane, which can lead to an augmented permeability of the erythrocyte membrane and hemolysis [17].
The blood transfusion service in Indonesia, either in the blood transfusion department of Indonesian Red Cross or in hospitals, has not yet established rules regarding smoking donors. In other words, smoking donors are not controlled yet, which, perhaps, encourages many active smokers to donate their blood with the beneficial consequences for their own hematopoiesis. Since free radicals can increase the oxidative stress in erythrocytes of a smoker, our study aims to investigate the correlation between oxidative stress and erythrocyte membrane resistance in stored leukoreduced PRC originating from active smokers. The leukocyte reduction method used in our study was designed to eliminate leukocytes that are higher in smokers than in nonsmokers, and can cause rapid free radical generation. Hence, our goal was to examine the effect of oxidative stress on the membrane resistance of stored leukoreduced PRC donated by active smokers.
Material and Methods
Subjects
Our research had cross-sectional design. It involved 36 donors at the Blood Transfusion Unit of the Indonesian Red Cross in Jakarta from September through November of 2020. The minimum number of collected samples was 12 from 36 donors for each group of donors: 12 nonsmoking (NS) donors, 12 light smokers (LS), and 12 moderate smokers (MS) (Table 1). Active smokers included 1) light smokers (1-10 cigarettes per day), 2) moderate smokers (11-20 cigarettes daily), and 3) smoking at least five years. The inclusion criteria used for this study were that the donors did not respond to human immunodeficiency virus (i.e., were HIV-negative), hepatitis C virus (HCV), hepatitis B surface antigen virus (HBsAg), and treponema pallidum hemagglutination assay (TPHA).
Table 1. Characteristics of study subjects
|
|
Non-smokers |
Light smokers |
Moderate smokers |
|||
|
|
Mean ± SD |
Range |
Mean ± SD |
Range |
Mean ± SD |
Range |
|
Age, years |
34.33±8.42 |
22-48 |
36.83±7.42 |
26-47 |
38.17±6.63 |
29-50 |
|
Time, years |
0 |
0 |
13.75±6.08 |
5-20 |
15.08±3.09 |
10-20 |

where: r is literature correlation (14-Norah A) = 0.0685 (p<0.05); Zα is standard deviation of alfa = 1.960; Zβ is standard deviation of beta = 0.842, power = 80%.
Leukoreduced PRC formation
Complete blood sampling from the subjects was carried out with a double pack of 450 mlL with CPDA-1 anticoagulant. Next, a 5000 g centrifugation at 4 ℃ was performed for 5 minutes to separate blood components and obtain PRC. Thereafter, the PRC was filtered using a Haemonetics Factory inline six-bag filter BPF4. Six packs of leukoreduced PRCs were labeled based on observation periods, starting from day 0 (D0), day 7 (D7), day 14 (D14), day 21 (D21), day 28 (D28) and day 35 (D35), and examined for MDA, SOD, osmotic fragility test (OFT) and hemolysis score. Finally, the leukoreduced PRC was stored in a blood refrigerator at 2–6 ℃.
MDA assessment
Hemolysate (400 μL) was mixed with 20% trichloroacetic acid. The mixture was homogenized and centrifuged at 5000 rpm for 10 minutes. In addition, 400 μL of 0.67% thiobarbituric acid (TBA) was added to the supernatant. The principles of operation were maintained during MDA condensation, in which the TBA was conditioned at a low pH level and a temperature of 96 ℃ for 10 min. TBA-generated pink chromogen can be identified spectrophotometrically using lambda at 532 nm. The amount of detected MDA correlates with the amount of lipid peroxide [18].
SOD assessment
The procedure was performed using RanSOD manufactured by RANDOX. The hemolysate was diluted 200 times and reacted with the mixed substrate and xanthine oxidase. Xanthine in a mixed substrate is converted by the arthritis enzyme, xanthine oxidase, yielding a superoxide radical (O2*) that reacts with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazoline chloride (INT) to form red supernatant. Furthermore, the activity of superoxide dismutase was measured in addition to the inhibition level of this reaction. A unit of SOD, resulting in the inhibition level of 50% of the reduction INT, was evaluated. The origin of the red color produced by the supernatant can be measured using a 505 nm lambda supernatant [19].
Assessment of red blood cell resistance
The supernatant method of OFT was used. A serial concentration of 0.1-0.8% NaCl was prepared in a 2 mL microtube, 1998 µl in size, with a 2 µL blood sediment. In addition, it was centrifuged at 1000 g for 15 minutes and the supernatant lambda of 540 nm. The supernatant resistance of erythrocytes was assessed based on their fragility in various concentrations of NaCl or hypoosmotic solutions. When the resistance of erythrocytes to the supernatant decreased, hemolysis easily occurred, which caused the release of Hb from erythrocytes. Thus, the Hb concentration could be measured in the supernatant with a wavelength of 540 nm [20]. The following formula was used to determine the percentage (%) of hemolysis:

A blank = NaCl concentration of 0.8%; A standard = NaCl concentration of 0.1%.
Statistical analyses
The collected data were analyzed using a nonparametric Kruskal-Wallis test, followed by Mann-Whitney test with a 5% margin of error. The relationship between variables (e.g., SOD, MDA, and membrane fragility) was analyzed using a nonparametric Spearman rank correlation test. The data were processed using IBM SPSS Statistics v.23 software.
Results
MDA in stored leukoreduced PRC on D0, D7, D14, D21, D28, and D35
According to the Kruskal-Wallis test, the medians of MDA in the NS, LS, and MS groups tended to increase with time of storage (from D0 to D35) and were significantly different (p<0.05) (Table 2). The MS group exhibited the highest median of MDA over the entire assessment period.
Table 2. Increase in MDA concentration in leukoreduced PRC
|
Day |
Subjects |
p1 |
NS vs. LS |
NS vs. MS |
||||||||
|
Non-smokers |
Light smokers |
Moderate smokers |
p2 |
p2 |
||||||||
|
Median |
Min |
Max |
Median |
Min |
Max |
Median |
Min |
Max |
||||
|
0 |
1.02 |
0.45 |
2.42 |
1.47 |
1.18 |
3.22 |
2.23 |
1.38 |
4.30 |
0.001 |
0.006 |
0.001 |
|
7 |
1.59 |
0.58 |
2.57 |
2.96 |
2.21 |
4.26 |
3.85 |
2.28 |
4.97 |
0.000 |
0.000 |
0.000 |
|
14 |
2.97 |
2.12 |
4.22 |
3.78 |
2.60 |
4.36 |
4.66 |
3.61 |
7.20 |
0.000 |
0.013 |
0.000 |
|
21 |
3.17 |
2.29 |
4.26 |
4.42 |
2.84 |
4.60 |
4.91 |
4.04 |
7.34 |
0.000 |
0.003 |
0.000 |
|
28 |
3.25 |
2.51 |
4.30 |
4.53 |
3.16 |
5.11 |
5.33 |
4.10 |
7.43 |
0.000 |
0.002 |
0.000 |
|
35 |
3.47 |
2.72 |
4.88 |
4.64 |
0.14 |
5.65 |
5.93 |
4.19 |
8.26 |
0.000 |
0.043 |
0.000 |
p1 is p value for Kruskal-Wallis test; p2 is p value for Mann-Whitney test.
SOD activity in stored leukoreduced PRC on D0, D7, D14, D21, D28, and D35
As shown in Table 3, the median SOD activity tended to decline and differed statistically significantly (sensu the Kruskal-Wallis test) between the NS, LS and MS groups over the entire storage period from D0 up to D35. However, the Mann-Whitney test performed for NS and MS exhibited different decreasing trends for SOD activity during storage on D0, D7, D14, D21, D28, and D35.
Table 3. Reduction in SOD activity in leukoreduced PRC
|
Day |
Subjects |
p1 |
NS vs. LS |
NS vs. MS |
||||||||
|
Non-smokers |
Light smokers |
Moderate smokers |
p2 |
p2 |
||||||||
|
Median |
Min |
Max |
Median |
Min |
Max |
Median |
Min |
Max |
||||
|
0 |
148.35 |
47.73 |
422.54 |
119.40 |
95.91 |
161.87 |
73.83 |
30.86 |
95.91 |
0.001 |
0.543 |
0.024 |
|
7 |
119.40 |
67.66 |
461.06 |
80.56 |
36.74 |
161.87 |
42.24 |
10.83 |
67.66 |
0.000 |
0.005 |
0.000 |
|
14 |
62.25 |
21.77 |
210.29 |
56.83 |
15.36 |
73.83 |
22.76 |
7.64 |
62.01 |
0.002 |
0.368 |
0.003 |
|
21 |
40.24 |
16.76 |
104.65 |
29.80 |
14.08 |
62.01 |
16.76 |
4.15 |
43.75 |
0.004 |
0.213 |
0.002 |
|
28 |
21.02 |
14.08 |
67.66 |
16.76 |
12.90 |
30.86 |
9.51 |
3.19 |
19.95 |
0.002 |
0.201 |
0.002 |
|
35 |
15.36 |
10.83 |
40.09 |
11.82 |
10.83 |
15.36 |
4.17 |
2.68 |
9.10 |
0.000 |
0.010 |
0.000 |
p1 is p value for Kruskal-Wallis test; p2 is p value for Mann-Whitney test.
Membrane resistance sensu OFT with 0.54% NaCl in stored leukoreduced PRC on D0, D7, D14, D21, D28, and D35
The percentage increase in erythrocyte membrane fragility in 0.54% NaCl solution, based on the Kruskal-Wallis test between the groups, amplified and differed statistically significantly from the beginning to the end of the storage period. In addition, the Mann-Whitney test for NS vs. LS exhibited significant differences. Comparison of NS with MS yielded similar results, indicating an increase in the percentage and significant differences throughout the entire blood storage period (Table 4).
Table 4. Percentages of membrane fragility increase in leukoreduced PRC for 0.54% NaCl solution
|
Day |
Subjects |
p1 |
NS vs. LS |
NS vs. MS |
||||||||
|
Non-smokers |
Light smokers |
Moderate smokers |
p2 |
p2 |
||||||||
|
Median |
Min |
Max |
Median |
Min |
Max |
Median |
Min |
Max |
||||
|
0 |
6.02 |
0.44 |
7.85 |
16.11 |
6.75 |
20.39 |
32.54 |
20.00 |
37.74 |
0.001 |
0.006 |
0.001 |
|
7 |
6.71 |
4.24 |
8.41 |
16.70 |
13.33 |
28.24 |
34.04 |
21.83 |
45.68 |
0.000 |
0.000 |
0.000 |
|
14 |
7.57 |
5.36 |
12.24 |
21.86 |
16.88 |
36.87 |
38.80 |
23.50 |
49.41 |
0.000 |
0.013 |
0.000 |
|
21 |
9.57 |
6.36 |
14.40 |
26.42 |
19.07 |
37.86 |
45.90 |
29.89 |
50.34 |
0.000 |
0.003 |
0.000 |
|
28 |
10.03 |
6.77 |
23.53 |
34.52 |
22.22 |
47.90 |
48.53 |
30.43 |
53.67 |
0.000 |
0.002 |
0.000 |
|
35 |
12.62 |
9.59 |
43.48 |
39.63 |
29.94 |
46.15 |
51.30 |
39.89 |
58.68 |
0.000 |
0.043 |
0.000 |
p1 is p value for Kruskal-Wallis test; p2 is p value for Mann-Whitney test.
Correlation between MDA concentration, SOD, and membrane fragility
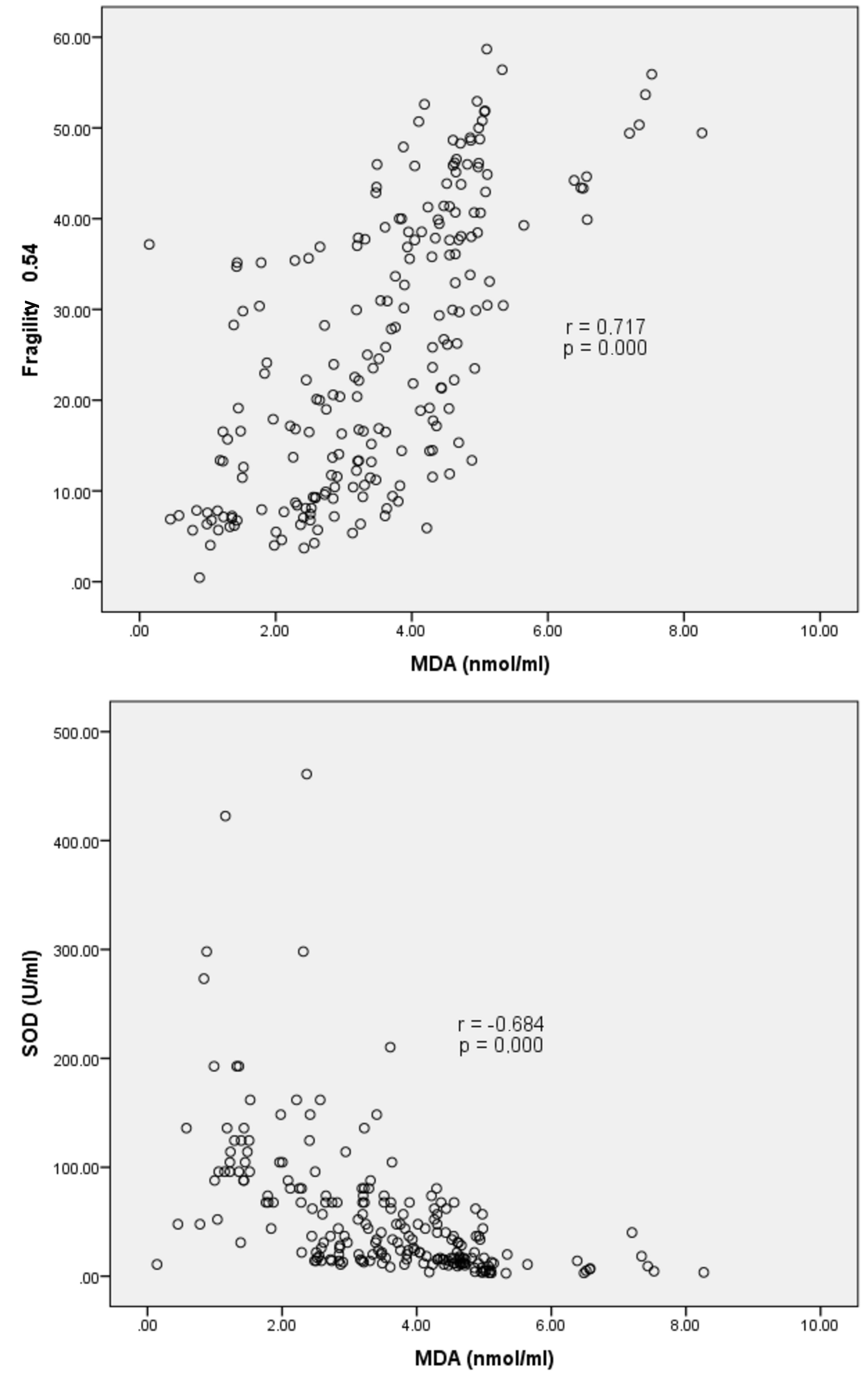
A strong negative statistically significant correlation between the concentration of MDA and SOD was confirmed by r=−0.684 and p=0.000 (Figure 1). This finding implied that at a higher MDA concentration, the activity of SOD was relatively lower. In addition, a strong positive significant correlation was observed between MDA and membrane fragility in 0.54% NaCl solution, confirmed by r=0.717 and p=0.000, which implied that at a higher MDA concentration, membrane fragility was also higher.
Figure 1. Correlation of MDA concentration with SOD and membrane fragility in 0.54% NaCl solution.
Relationship between SOD activity, membrane fragility and hemolysis
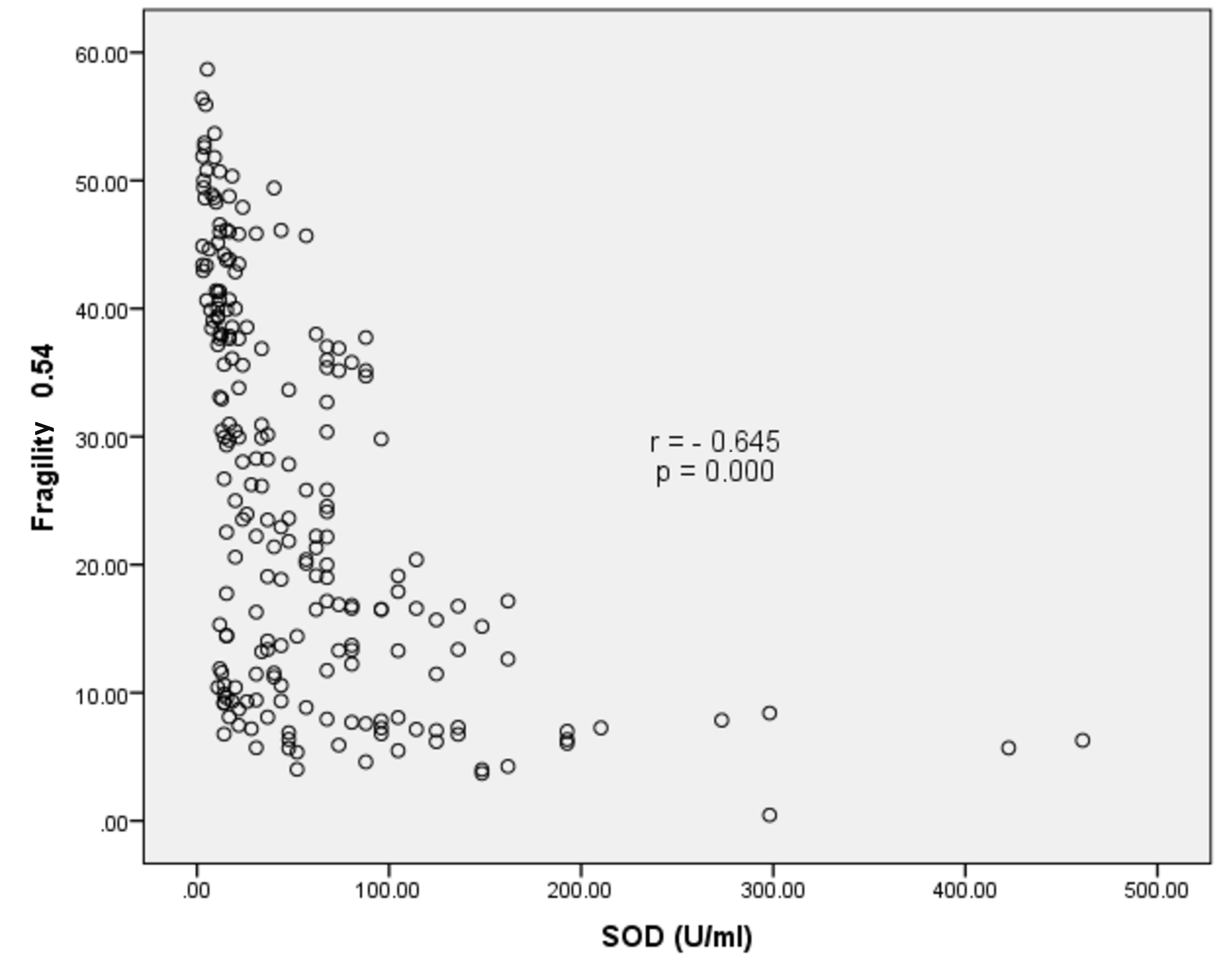
A strong significant negative correlation was observed between SOD activity and membrane fragility with p=0.000 and r=−0.645. This implied that membrane fragility was relatively lower at higher SOD levels (Figure 2).
Figure 2. Correlation of SOD activity with membrane fragility in a 0.54% NaCl solution.
Discussion
In these three groups, i.e., NS, LS and MS, we established an increase in the concentration of MDA and a decrease in the activity of SOD on D0-D35 of the assessment. This was indicative of higher oxidative stress during storage of PRC with reduced leukocyte content at the end of the storage period, showing that the MS group had the highest level of oxidative stress, followed sequentially by the LS and NS groups. During storage, erythrocyte metabolism continued, causing storage lesions, one of which was Hb oxidation. Hb oxidation/ or Hb autoxidation generated free radicals. Free radicals would continue to be produced during Hb autoxidation, yielding superoxide and hydrogen peroxide, both of which were the source of the oxidative reaction [21].
Moreover, the erythrocyte membrane containing cholesterol, phospholipids and PUFAs was more readily exposed to free radical penetration. Free radicals were destroying the double bond in the PUFA of the bilayer membrane and forming unstable lipid peroxide compounds. The lipid peroxide eventually formed the MDA compound. The process of free radical formation occurred continuously until the end of the PRC shelf life, which led to an increase in the concentration of MDA. An increase in MDA concentration implied progressive damage that could cause a decrease in fluidity and an increase in the permeability of erythrocyte membranes, causing a reduction in erythrocyte membrane resistance prior to the hemolysis [22]. The study carried out by Korgun et al. (2001) showed that the content of lipid peroxide in PRC increased and the content of antioxidants decreased during the storage. In nature, the decrease in the content of antioxidants in erythrocytes depended on the duration of storage. According to Aslan et al. (1997), there was a significant increase in the concentration of MDA (viz., on D3-D19), followed by a significant reduction in SOD activity, based on the assessment on D13 of the storage [23]. Marjani et al. (2007) argued that during red blood cell storage, the MDA concentration increased on D9, and the activity of SOD enzyme declined on D11 [24]. Ogunro et al. (2010) confirmed an increase in MDA activity by 24.8% and a decrease in SOD activity by 17.1% when assessing red blood cells on D20 using storage with anticoagulant (CPDA-1) [25].
Smoking donors may have aggravated oxidative stress in leukoreduced PRC due to free radicals in their erythrocytes, i.e., superoxide anion, hydrogen, hydroxyl, and peroxyl [26]. Presumably, a puff of cigarette smoke contained 10 free radicals [14, 15, 27]. The number of smoked cigarettes and the duration of smoking contributed to the excessive formation of free radicals in the body, which was accompanied by a higher concentration of lipid peroxide and MDA [14, 26]. Moreover, lipid peroxide broke down erythrocyte membrane PUFAs by changing lipid fluidity in the central bilayer zone, yielding cell membrane rigidity, osmotic fragility, and reduced lipid fluidity [28, 29].
Physiologically, free radicals are suppressed by SOD enzymes as endogenous antioxidants present in red blood cells. In fact, SOD prevent oxidants by catalyzing the reaction of superoxide anion dismutase into hydrogen peroxide and oxygen molecules [14]. In erythrocytes obtained from smoking donors, an increase in hydrogen peroxide was observed, formed as a result of direct interaction of smoke with tissues, which significantly changed the activity of the SOD enzymes [25]. Similar to the study carried out by Norah et al. (2019), the number of cigarettes smoked per day (1-5, 6-10, or >10) positively correlated with the concentration of MDA in erythrocytes. This showed that those who consumed >10 cigarettes per day would have a higher concentration of MDA and less pronounced SOD activity than the other two groups of study subjects [14].
In addition, oxidative stress affected Cord 3, which was associated with the rapid aging of erythrocytes due to oxidized hemoglobin. This modification limited the ability of erythrocytes to maintain the biconcave shape used to pass through the capillary. Moreover, oxidative stress was one of several factors causing cell shrinkage, which as possibly due to potassium leakage through the Gardos channel. Preceding damage to Ca2+-ATPase, which maintained a low concentration of intracellular calcium ion, caused an increase in intracellular calcium ion. An increase in intracellular calcium activated the Gardos channel, promoting potassium leakage, deterioration of deformability, and red blood cell shrinkage [30]. Besides, Na+-K+ATPase pumping activity was disabled, as it was affected by temperature and cell metabolism. However, cell metabolism continued during the storage of leukoreduced PRC. According to Oppoku-Oprah (2015), the temperature of 4 ℃ and the state of high lactic acid accumulation due to cellular glycolysis made Na+-K+ATPase pumping inactive. This event stimulated sodium to enter the cells while potassium exits the cells. Although this was happening gradually, it went steadily during storage. Furthermore, intracellular sodium would become excessive, and potassium would accumulate in cells, causing inflammation in erythrocytes and membrane fragility in lysed cells [31].
Cigarette smoke contains oxygen, carbon, and oxygen radicals, causing the formation of macrophages in alveolar cells and generating active oxygen radicals. Besides, the cigarette smoke tar can reduce oxygen and produce superoxide. ROS in cigarette smoke serve to generate peroxyl radicals and can lead to lipid peroxidation in erythrocytes. Superoxide and hydroxyl radicals could cause cell damage [29]. Carbon monoxide with a strong affinity for Hb will construct HbCO2, which will also cause red blood cell lysis. Nicotine causes damage to red blood cells as well, their destruction and lysis [27]. Nora et al. (2019) showed that smoking duration strongly and positively correlated with an increase in MDA concentration, while strongly and negatively correlated with a decrease in SOD activity [14]. In other words, as the duration of smoking increased, the numbers of free radicals in the body also became higher due to conventional smoke containing gaseous and solid phases. Both of these phases were found to be rich in free radical sources and could increase cell damage, because the occurrence of oxidative stress was relatively high.
It was stated in the literature that hemolysis in a 0.54% NaCl solution was 0%, while blood in which 70–80% of all blood cells remained viable 24 h after transfusion was called high-quality blood [32]. Our study demonstrated that compared to the NS and LS groups, the MS group had the highest level of increase in membrane fragility to 0.54% NaCl solution. In the NS group, measurement of membrane fragility on D35 showed that 17.53% + 12.16% of erythrocytes underwent hemolysis, which meant that approximately 82.47% of leukoreduced PRC were relatively viable. At the same time, hemolysis reached 34.10% + 7.95% in the LS group, which meant that only approximately 65.9% of red blood cells were viable. Finally, in the MS group, hemolysis already occurred on D0 (30.92% + 5.98%). This finding meant that there were just 69.08% of viable blood cells on day 0, originating from moderately smoking donors.
Study limitations
Small sample sizes were one of the present study limitations, as blood donations received from PMI DKI Jakarta were severely limited due to a significant reduction in donors during the COVID-19 pandemic. Our data were not normally distributed, so they could not be processed using any parametric analysis.
Conclusions
We established augmented MDA concentration, membrane fragility, and hemolysis in leukoreduced PRC during the 35-day storage period in the NS, LS, and MS groups, with the highest increase detected in the MS group. Additionally, SOD enzyme activity somewhat declined in leukoreduced PRC during 35 days of storage in the NS, LS, and MS groups, with the biggest decrease exhibited by the MS group. Furthermore, erythrocyte membrane fragility correlated with oxidative stress. Therefore, the blood components of leukoreduced PRC could be used for repeated transfusions until D35 of their storage in the blood bank in nonsmoking donors and until D21 of storage in light smokers. As for moderate smokers among donors, their blood components of leukoreduced PRC should be banned for repeated transfusions. Further research regarding the contribution of oxidative stress to membrane cell resistance in conventional (non-leukoreduced) PRC in actively smoking donors is of a particular importance.
Acknowledgments
Universitas Indonesia PUTI Saintekes Grant number NKB-4652/UN2.RST/HKP.05.00/2020 and Blood Transfusion Unit of province DKI Jakarta, Indonesia
Conflict of interest
Authors declared no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments, or comparable ethical standards. The study was licensed by the Committee of Health Research Ethics, School of Medicine, University of Indonesia, with a reference number of 20-02-0247.
- Order of the Minister of Healthcare of the Republic of Indonesia No. 91 of 2015. Standards for Blood Transfusion Service. Jakarta. Indonesian. https://www.regulasip.id/book/5018/read
- Raat NJ, Verhoeven AJ, Mik EG, Gouwerok CW, Verhaar R, Goedhart PT, et al. The effect of storage time of human red cells on intestinal microcirculatory oxygenation in a rat isovolemic exchange model. Crit Care Med 2005; 33(1): 39-45. https://doi.org/10.1097/01.ccm.0000150655.75519.02.
- Wahidiyat PA, Adnani NB. Rational blood transfusion in children. Sari Pediatrik 2016; 18(4): 325-331. Indonesian. https://saripediatri.org/index.php/sari-pediatri/article/view/448/pdf.
- Yawata Y. Composition of Normal Red Cell Membranes. In: Yawata Y. Cell Membrane: The Red Blood Cell as a Model. Wiley‐VCH Verlag GmbH & Co. KgaA. 2003: 27-46. https://doi.org/10.1002/3527601538.ch2.
- D’Alessandro A, Kriebardis AG, Rinalducci S, Antonelou MH, Hansen KC, Papassideri IS, et al. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion 2015; 55(1): 205-219. https://doi.org/10.1111/trf.12804.
- Hoffbrand AV, Moss PAH. Essential Haematology. 7th ed. Chichester: John Wiley & Sons. 2016; 384 p. https://www.wiley.com/en-ie/Hoffbrand%27s+Essential+Haematology%2C+7th+Edition-p-9781118408636.
- Hertfelder H, Süwer V, Popov-Cenic S, Tschesche H, Hanfland P. Leukocyte proteinase release during storage of red cell concentrates. Eur J Clin Chem Clin Biochem 1994; 32(6): 441-447. https://doi.org/10.1515/cclm.1994.32.6.441.
- Pedersen KM, Çolak Y, Ellervik C, Hasselbalch HC, Bojesen SE, Nordestgaard BG. Smoking and increased white and red blood cells. Arterioscler Thromb Vasc Biol 2019; 39(5): 965-977. https://doi.org/10.1161/atvbaha.118.312338.
- Boehm R, Cohen C, Pulcinelli R, Caletti G, Balsan A, Nascimento S, et al. Toxic elements in packed red blood cells from smoker donors: A risk for paediatric transfusion? Vox Sanguinis 2019; 114(8): 808-815. https://doi.org/10.1111/vox.12854.
- Sundari R, Widjaya DS, Nugraha A. Duration of smoking and effect of number of cigarette consumption on platelets in active male smokers. Kesmas: J Kesehat Masy Nas (Nat Public Health J) 2015; 9(3): 257-263 Indonesian. http://doi.org/10.21109/kesmas.v9i3.692.
- Boehm RE, Do Nascimento SN, Cohen CR, Bandiera S, Pulcinelli RR, Balsan AM, et al. Cigarette smoking and antioxidant defenses in packed red blood cells prior to storage. Blood Transfus 2020; 18(1): 40-48. https://doi.org/10.2450/2019.0166-19.
- Kesuma Y, Yenrina R. Understanding Free Radicals. In: Kesuma Y, Yenrina R. Natural and Synthetic Antioxidants. Padang: Andalas University Press. 2015: 15-16. Indonesian. http://repository.unand.ac.id/23714.
- Carnevale R, Cammisotto V, Pagano F, Nocella C. Effects of smoking on oxidative stress and vascular function. In: Smoking Prevention and Cessation, M Rajer, ed. IntechOpen, 2018: 25-47. http://doi.org/10.5772/intechopen.78319.
- Norah A, Okaforchidimma, Dioka C, Meludu S. Evaluation of total antioxidant status, superoxide dismutase and malondialdehyde in apparently healthy active tobacco smokers in Nnewi Metropolis, South East Nigeria. J Sci Innov Res 2017; 6(3): 105-112. https://www.jsirjournal.com/Vol6_Issue3_06.pdf.
- Bray RC, Cockle SA, Fielden EM, Roberts PB, Rotilio G, Calabrese L. Reduction and inactivation of superoxide dismutase by hydrogen peroxide. Biochem J 1974; 139(1): 43-48. https://doi.org/10.1042/bj1390043.
- Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014; 2014: 360438. https://doi.org/10.1155/2014/360438.
- Wolfe LC. The membrane and the lessions of storage in preserved red cells. Transfusion 1985; 25 (3): 185-203. https://doi.org/10.1046/j.1537-2995.1985.25385219897.x.
- Olszewska-Słonina DM, Mątewski D, Czajkowski R, Olszewski KJ, Woźniak A, Odrowąż-Sypniewska G, et al. The concentration of thiobarbituric acid reactive substances (TBARS) and paraoxonase activity in blood of patients with osteoarthrosis after endoprosthesis implantation. Med Sci Monit 2011; 17(9): CR498-CR504. https://doi.org/10.12659/msm.881936.
- Monza RX. RANSOD Superoxide Dismutase Manual Vol.50. Antrim: RANDOX Laboratories Ltd. 2009: 26-28. https://www.tokyofuturestyle.com/pdf/randox_RANSOD.pdf.
- Walski T, Chludzińska L, Komorowska M, Witkiewicz W. Individual osmotic fragility distribution: A new parameter for determination of the osmotic properties of human red blood cells. Biomed Res Int 2014; 2014: 162102. https://doi.org/10.1155/2014/162102.
- Rifkind JM, Mohanty JG, Nagababu E. The pathophysiology of extracellular hemoglobin associated with enhanced oxidative reactions. Front Physiol 2015; 5: 500. https://doi.org/10.3389/fphys.2014.00500.
- Vani R, Soumya R, Manasa K, Carl H. Storage lesions in blood components. Oxid Antioxid Med Sci 2015; 4(3): 125-132. https://doi.org/10.5455/oams.130915.rv.019.
- Aslan R, Sekeroğlu MR, Tarakçioğlu M, Köylü H. Investigation of malondialdehyde formation and antioxidant enzyme activity in stored blood. Haematologia (Budap) 1997; 28(4): 233-237. https://pubmed.ncbi.nlm.nih.gov/9408767.
- Marjani A, Moradi A, Ghourcaie AB. Alterations in plasma lipid peroxidation and erythrocyte superoxide dismutase and glutathione peroxidase enzyme activities during storage of blood. Asian J Biochemistry 2007; 2(2): 118-123. https://doi.org/10.3923/ajb.2007.118.123.
- Ogunro PS, Ogungbamigbe TO, Muhibi MA. The influence of storage period on the antioxidants level of red blood cells and the plasma before transfusion. Afr J Med Med Sci 2010; 39(2): 99-104. https://pubmed.ncbi.nlm.nih.gov/21117405.
- Garg N, Singh R, Dixit J, Jain A, Tewari V. Levels of lipid peroxides and antioxidants in smokers and non-smokers. J Periodont Res 2006; 41(5); 405-410. https://doi.org/10.1111/j.1600-0765.2006.00889.x.
- Antonio P. Effect of Smoking on Quality of Red Blood Cells Collected from Donors Who are Smokers and Stored for a Period of 35 Days in the Blood Bank. Master’s dissertation. Vellore: Christian Medical College; 2018; 135 p. http://repository-tnmgrmu.ac.in/9485.
- Bray RC, Cockle SA, Fielden EM, Roberts PB, Rotilio G, Calabrese L. Reduction and inactivation of superoxide dismutase by hydrogen peroxide. Biochem J 1974; 139(1): 43-48. https://doi.org/10.1042/bj1390043.
- Pannuru P, Vaddi DR, Kindinti RR, Varadacharyulu N. Increased erythrocyte antioxidant status protects against smoking induced hemolysis in moderate smokers. Hum Exp Toxicol 2011; 30(10): 1475-1481. https://doi.org/10.1177/0960327110396527.
- Mohanty JG, Nagababu E, Rifkind JM. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front Physiol 2014; 5: 84. https://doi.org/10.3389/fphys.2014.00084.
- Opoku-Okrah C, Acquah BKS, Dogbe E. Changes in potassium and sodium concentrations in stored blood. Pan Afr Med J 2015; 20: 236. https://doi.org/10.11604/pamj.2015.20.236.5851.
- Harmening DM, Lasky L, Latchaw P. Blood preservation: historical perspectives, review of metabolism and current trends. In: Harmening DM, editor. Modern blood banking and transfusion practices. 4th ed. Philadelphia, PA: F.A. Davis co.; 1999. https://books.google.co.id/books?hl=en&lr=&id=vxyDDwAAQBAJ&oi=fnd&pg=PR1&ots=-RIXOmAvJr&sig=M63Oqz7MEkXUye89IWZ4W8x7ghE&redir_esc=y#v=onepage&q&f=false
Received 14 February 2022, Revised 2 April 2022, Accepted 17 May 2022
© 2022, Russian Open Medical Journal
Correspondence to Sri Widia A. Jusman. Address: Department of Biochemistry and Molecular Biology, School of Medicine, Universitas Indonesia, 4 Salemba Raya St., Jakarta 10430, Indonesia. Phone: +628569039969, +6281327988851. E-mail: sriwidiaaj@gmail.com, maildeas.uddpmi@gmail.com.