Introduction
In Wuhan, China, in December 2019, cases of severe atypical viral pneumonia infection emerged, spreading swiftly and causing a significant number of deaths [1]. Subsequently, the virus genome was sequenced, determining that it was a new coronavirus called SARS-CoV-2 [2]. On March 11, 2020 the World Health Organization declared COVID-19 a pandemic, affecting more than 180 countries around the world [3]. By now, it has caused over 114,518 deaths worldwide, and more than 5.2 million people have been infected in our country (https://coronavirus.jhu.edu/map.html). This disease is considered at present an international public health emergency. This has forced countries to adopt various policies with the goal of fighting the pandemic. According to recent reports, most COVID-19 patients have an incubation period of 3 to 7 days [4]. Fever, cough, and fatigue are the most common symptoms, while nasal congestion, discharge, and diarrhea are only seen in a small portion of patients [5]. Severe cases can rapidly progress to acute respiratory distress syndrome (ARDS), septic shock, metabolic acidosis, and bleeding due to coagulation dysfunction [6]. Health care community is making every effort to provide adequate treatment to patients, limit the spread of the virus and overcome this pandemic; hence, a well-timed and accurate diagnosis of SARS-CoV-29 infection is essential [7]. In this sense, detection of viral RNA based on RT-PCR is the gold-standard to confirm the diagnosis of SARS-CoV-2 infection. Regarding prophylaxis, at present, more than 187 vaccines against COVID-19 are being developed, 44 of which are in various stages of human clinical trial testing (8). Recently, the first results of early phase clinical trials, which have tested 9 candidate vaccines, have been published. Tested vaccines represent a wide range of platform technologies including mRNA vaccines, such as Moderna [9] and Pfizer/BioNTech [10], adenoviral vector-based vaccines as per CanSino [11], Oxford [12], Gamaleya Research Institute of Russia [13] and Janssen/Beth Israel [14]; and a recombinant protein vaccine with Novavax adjuvant [15] and two inactivated virus vaccines [16, 17]. Recombinant adenovirus (rAd)-based vaccines, such as Sputnik V, are suitable candidates for the long-term protection of people at high risk of COVID-19 since they stimulate the rapid onset of protective immunity. Gam-COVID-Vac (Sputnik V) is a combined vector vaccine, based on rAd type 26 (rAd26) and rAd type 5 (rAd5), both carriers of the full-length SARS-CoV-2 glycoprotein S gene (rAd26-S and rBd5-S). RAd26-S and rAd5-S are administered intramuscularly separately with an interval of at least 21 days. Overall, trial results suggested that vaccination was relatively safe and reasonably well tolerated. Importantly, all vaccination modalities induced detectable antibody titers by ELISA, as well as variable neutralizing antibody (nAb) titers levels. The vast majority of these vaccines were developed with the aim of inducing nAb. However, there is still no evidence of the nAb magnitude required for protection or the durability of nAb responses. The goalof our study was to assess the humoral response (antibody levels) in a health worker population after being vaccinated with the first and second doses of Sputnik V.
Material and Methods
Studied population
This study included 530 health workers in the city of Buenos Aires vaccinated with Sputnik V. Within this population, 25% (n=133) had already suffered from Covid infection prior to their vaccination. This vaccine is based on heterologous recombinant adenovirus (rAd), Gam-COVID-Vac (Sputnik V) [18]. Our study used a longitudinal observational design.
Measuring total SARS-CoV-2 antibodies
SARS-CoV-2 total antibodies (IgG and IgM) were measured in serum samples, using a semi-quantitative chemiluminescent assay in the Centaur XPT autoanalyzer, Siemens®. This system reports assay results by index values with a measurement range from 0.05 to 10. The outcome is considered negative for SARS-CoV-2 antibodies when the index is <1.0 (non-reactive), and positive when the index is >1.0 (reactive). Antibody levels were measured after the first dose and after the second dose of Sputnik V vaccine.
Statistical analyses
The distribution of variables was identified using normality tests (kurtosis and skewness). Results were expressed as mean ± standard deviation (SD) or median (range), depending on the data distribution. Mean and median differences were evaluated by t-test or Mann-Whitney test, respectively. Statistical analyses were performed using the SPSS 19.0 program (Chicago, IL).
Ethical standards
The participants did not receive any kind of compensation for participating in the study. The study was approved beforehand by the Ethics Committee of the Hospital and was performed in compliance with the Declaration of Helsinki and its later amendments for medical studies involving human subjects.
Results
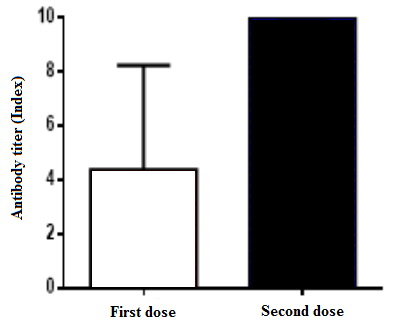
From a total of 530 individuals vaccinated with Sputnik V, 25% were infected with COVID-19 (n = 133), confirmed by the real-time PCR (Table 1). Table 2 demonstrates the levels of antibodies obtained after the application of the first and second doses of Sputnik V, according to the elapsed days. Table 3, along with Figures 1 and 2, show the antibody levels in vaccinated individuals who did not have COVID-19 and in individuals who received the vaccine after being infected with the virus. After 21 days of the first dose application, 10 subjects (1.9%) exhibited antibody levels <1.0 (non-reactive) and 520 subjects (98.1%) had a reactive humoral response (>1.0). The results 21 days after the second dose showed that only 2 individuals (0.38%) had antibody levels <1.0 (non-reactive), while 528 subjects (99.6%) responded with antibody values >1.0 (reactive).
Table 1. Distribution of the total population included in the study and antibody levels
|
Parameters |
Study group |
|
Age (years) |
45±14 |
|
Gender % (n) |
F: 58,7 (313) M: 41,3 (220) |
|
Covid (n) |
133 |
|
Sputnik V (n) |
530 |
|
Covid+Sputnik (n, %) |
133 (25%) |
|
Days since 1st dose |
38±21 |
|
Total antibodies after the 1st dose |
4.4 (0.18–>10) |
|
Days since 2nd dose |
24±16 |
|
Total antibodies after the 2nd dose |
>10 (1.3–>10) |
Table 2. Antibody levels after the first and second dose
|
|
Days |
Total antibodies |
|
Days after the 1st dose |
0-20 |
1.5 (0.13–>10)* |
|
≥21 |
5.67 (0.05–>10)** |
|
|
Days after the 2nd dose |
0-20 |
>10 (1.3 –>10)+ |
|
≥21 |
>10 (<1.0–>10)++ |
Table 3. Post-vaccination antibody levels in the population with and without COVID-19 infection
|
|
Days since 1st Sputnik V dose |
Days since 2nd Sputnik V dose |
Total antibodies post 1st dose |
Total antibodies post 2nd dose |
|
Post Sputnik V |
39±20 |
23±15 |
2.76 (0.3-8.3)1 |
>10 (1.4–>10)2 |
|
Covid-19 +Sputnik V |
34±18 |
25±14 |
>10 (1.1–>10) |
>10 (5.8–>10) |
Mann Whitney Test 1vs2 p<0.0001.
Figure 1. Antibody levels in patients who were not infected by COVID-19.
Figure 2. Antibody levels in patients infected by COVID-19.
Discussion
Our results demonstrated that the two-component Gam-COVID-Vac (Sputnik V) vaccine was able to induce a humoral response in the individuals included in this study. Previous studies showed that this vaccine had an efficacy of 91.6% (95% CI 85.6-95.2) against COVID-19 in vaccinated patients, starting 21 days after the application of the first dose.
In our research, we observed that after the first dose, 98.1% of individuals generated antibodies, while after the second dose, such effect was detected in 99.6% of cases. Logunov et al [12] found that the vaccine induced strong humoral responses in all age ranges; however, in a few cases, no response was observed (6 of 342). There is strong evidence that many factors influence the immune response to vaccination. Among them, we should mention intrinsic factors (genetics, gender, age at time of vaccination, and comorbidities); extrinsic factors (infections, microbiota and antibiotics); behavioral factors (smoking, exercise, alcohol consumption, stress, and sleep quality); nutritional factors (body mass index, nutrition status, micronutrients, enteropathy); and environmental factors (geographic location, season, toxins, family size). Also, vaccine-related aspects are involved, such as choice of vaccine products, adjuvants, and vaccination schedule [19].
The lack of antibody rise in this small group could be due to immunosenescence in the elderly, individual characteristics of the immune response, or concomitant immune disorders.
In a study carried out in Argentina by the Ministerio de Salud de la Provincia de Buenos Aires, in which 288 individuals vaccinated with Sputnik V were included, the results suggested that after 21 days of the vaccine first dose application, 94% of subjects developed specific IgG antibodies against spike protein, whereas 21 days after the second dose, 100% of them have developed such antibodies. In our study, we also observed that previously infected individuals with COVID-19 showed a more pronounced increase in antibodies after the first dose than those who were not previously infected: >10 (1.1–>10) vs. 2.76 (0.3–8.3). On the other hand, after the second dose, in both groups, the levels of antibodies were >10 (5.8–>10) vs. >10 (1.1–>10). These results are in agreement with recent studies carried out with the Pfizer and Moderna vaccines, which showed that the increase in the levels of antibodies after the first dose in people who had a previous exposure to the virus were very high and that the second dose did not generate further increase [16].
Conclusion
Our results demonstrated that Sputnik V vaccine was able to induce a humoral response against the spike protein of SARS CoV-2 in 99.6% of the individuals studied 21 days after the application of the second dose. This study suggested that two doses of Sputnik V vaccine triggered a proper antibody response in virtually the entire studied population. The second Sputnik V dose had no impact on IgG response for those who had previous exposure to SARS CoV-2.
Limitations of the study
There are some limitations in our study. One limitation is that demographic data from volunteers was not available, such as height, weight, or BMI. Another limitation is related to the fact that our antibody level results were reported as an index that had a maximum of 10, which could be slightly constrained; however, this is an inherent limitation of the method available at the time.
Conflict of interest
None declared.
- WHO. COVID-19 – China. 2020. https://www.who.int/emergencies/disease-outbreak-news/item/2020-DON233.
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al, China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382(8): 727-733. https://doi.org/10.1056/nejmoa2001017.
- COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). https://coronavirus.jhu.edu/map.html.
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al; China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382(18): 1708-1720. https://doi.org/10.1056/nejmoa2002032.
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020; 395(10223): 507-513. https://doi.org/10.1016/s0140-6736(20)30211-7.
- Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, et al. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin Infect Dis 2020; 71(16): 2027-2034. https://doi.org/10.1093/cid/ciaa344.
- Grigoryan L, Pulendran B. The immunology of SARS-CoV-2 infections and vaccines. Semin Immunol 2020: 50: 101422. https://doi.org/10.1016/j.smim.2020.101422.
- Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, et al. An mRNA vaccine against SARS-CoV-2 – Preliminary report. N Engl J Med 2020; 383(20): 1920-1931. https://doi.org/10.1056/nejmoa2022483.
- Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020; 586(7830): 589-593. Erratum in: Nature 2021; 590(7844): E26. https://doi.org/10.1038/s41586-020-2639-4.
- Zhu FC, Li YH, Guan XH, Hou LH, Wang WJ, Li JX, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020; 395(10240): 1845-1854. https://doi.org/10.1016/s0140-6736(20)31208-3.
- Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020; 396(10249): 467-478. Erratum in: Lancet 2020; 396(10266): 1884. https://doi.org/10.1016/s0140-6736(20)31604-4.
- Logunov DY, Dolzhikova IV, Zubkova OV, Tukhvatullin AI, Shcheblyakov DV, Dzharullaeva AS, et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet 2020; 396(10255): 887-897. https://doi.org/10.1016/s0140-6736(20)31866-3.
- Sadoff J, Le Gars M, Shukarev G, Heerwegh D, Truyers C, de Groot AM, et al. Interim results of a phase 1-2a trial of Ad26.COV2.S Covid-19 vaccine. N Engl J Med 2021; 384(19): 1824-1835. https://doi.org/10.1056/nejmoa2034201.
- Keech C, Albert G, Cho I, Robertson A, Reed P, Neal S, et al. Phase 1-2 Trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med 2020; 383(24): 2320-2332. https://doi.org/10.1056/nejmoa2026920.
- Xia S, Duan K, Zhang Y, Zhao D, Zhang H, Xie Z, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: Interim analysis of 2 randomized clinical trials. JAMA 2020; 324(10): 951-960. https://doi.org/10.1001/jama.2020.15543.
- Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis 2021; 21(1): 39-51. https://doi.org/10.1016/s1473-3099(20)30831-8.
- Informe Final I (Enero‐Marzo 2021) Empleo de la vacuna SputnikV en Argentina: Evaluación de respuesta humoral frente a la vacunación . 2021. Spanish. https://www.conicet.gov.ar/wp-content/uploads/Informe-Sputnik_Buenos-Aires-13.04.2021..pdf.
- Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, et al. Gam-COVID-Vac Vaccine Trial Group. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021; 397(10275): 671-681. https://doi.org/10.1016/s0140-6736(21)00234-8.
- Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev 2019; 32(2): e00084-18. https://doi.org/10.1128/cmr.00084-18.
Received 4 April 2022, Revised 11 October 2022, Accepted 23 November 2022
© 2022, Russian Open Medical Journal
Correspondence to Bibiana Fabre. Addresse: Junín 956 (C1113AAD), University of Buenos Aires. Facultad de Farmacia y Bioquímica. Departamento de Bioquímica Clínica. Buenos Aires, Argentina. Phone: +541159508654. Fax: +54115950 8691. E-mail: brfabre2000@yahoo.com.ar.