Introduction
Main neuronal mechanisms of coordination and integrative activity are inhibition processes in the spinal cord that have an important functional significance in the regulation of motor activity and locomotion [1-3]. Reflex mechanisms in the system of antagonist muscles are carried out with direct participation of reciprocal and presynaptic inhibition, and in the system of synergistic muscles, with nonreciprocal inhibition, also known as Ib inhibition, and recurrent inhibition via Renshaw cells [1].
An ultimate spinal nerve pathway in motion control is the disynaptic reciprocal inhibition of agonist and antagonist muscles [2, 3]. The afferents of Ia reciprocal inhibition not only form excitatory monosynaptic connections with homonymous α-motoneurons in the combination of stretch reflex arcs, but also coordinate reciprocal inhibitory connections with motoneurons of antagonist muscles, implemented via Ia inhibitory interneuron. The functional significance of reciprocal inhibition lies in timely coordinated work of flexor and extensor muscles in the course of performing various movements [2, 3].
The spinal system of presynaptic inhibition restricts the excessive flow of afferent signals to nerve centers and has principal biological significance in the processing of these signals in the central nervous system. Presynaptic inhibition is associated with primary afferent depolarization (PAD), mediated in axo-axonic synapses, and involves modification of a transmitter release in the synapse of Ia afferents to α-motoneuron using GABAA receptors, which sequentially increases the output of Cl- ions and generates polarization of afferent terminals [4]. Presynaptic inhibition regulates excess skeletal muscle tone, which prevents voluntary movements [5] and posture maintenance [6], and also actively regulates excess afferent inflow to the motor centers of the agonist and antagonist muscles of the lower leg, disinhibiting nonreciprocal and reciprocal inhibitory effects on them, thereby providing normal motor activity of a person [7].
In the last decade, a large number of experimental studies were published on the use of noninvasive transcutaneous electrical stimulation of the spinal cord (tESCS) [8-12]. This type of exposure is the application of electrical stimulation by means of electrodes attached to the lower thoracic and/or lumbosacral vertebrae. A distinctive feature of this technique is the use of painless stimulation modes on the spinal cord [8, 9]. Previously, we have demonstrated postactivation effects of 20-minute electrical stimulation of the spinal cord on the manifestation of nonreciprocal and recurrent inhibitory interaction in the system of agonist muscles in healthy individuals [12]. Previously unknown patterns have been established of the effect of a long-term electrical stimulation of the spinal cord on increasing muscle strength capabilities, along with a modulation of Ib interneurons of nonreciprocal inhibition, which ensures optimal maintenance of skeletal muscle tension [11, 13]. However, the reflex mechanisms of the tESCS action on the functional activity of reciprocal and presynaptic inhibition in the system of antagonist muscles in healthy subjects have not been studied.
An innovative feature of tESCS is its practical application in rehabilitation of patients with movement disorders due to diseases and injuries of the spinal cord [14-17]. Impairment of supraspinal and suprasegmental control of inhibitory systems is directly related to inability of performing a normal voluntary movement, which implies high functional significance of inhibitory interneuron circuits of the spinal cord in motion control [1, 18].
Consequently, the objective of our research was to examine an effect of a 20-minute electrical stimulation session of the spinal cord on manifestation of reciprocal and presynaptic inhibition in the system of shin antagonist muscles of healthy subjects in a state of relative muscle rest and weak muscle effort, along with possible physiological mechanisms of these manifestations.
Material and Methods
The study was conducted on the basis of Research Institute of Sports Problems and Health-Improving Physical Culture of Velikiye Luki State Academy of Physical Culture and Sports. The study involved 10 healthy men 27 to 35 years of age. All study participants were informed about the course of the experiment and gave written informed consent to participate in the study.
tESCS methodology
We performed the tESCS (Neuro-MVP-8 stimulator, Neurosoft LLC, Ivanovo, Russia) using an active electrode – specifically, a cathode of circular shape with a diameter of 2.5-3 cm and an adhesive conductive layer, which was attached along the midline of the vertebral column between the spinous processes of the thoracic vertebrae T11-T12 (Figure 1A) [9]. Subjects were placed on a couch in a supine position. Transcutaneous electrical stimulation of the spinal cord was performed for 20 minutes. Indifferent electrodes were anodes of a rectangular shape, 5×10.2 cm2, with an adhesive conductive layer; they were located bilaterally, symmetrically, above the iliac crests. The stimulus intensity during the first 10 minutes of the stimulation effect was within 30 mA, and later achieved 40 mA. The duration of a single stimulus was 0.5 ms, and the frequency of repeating the stimuli was 10 Hz [13].
Figure 1. Schematic description of the techniques. A. The tESCS technique; B. The technique of recording reciprocal and presynaptic inhibition mediated by Ia interneurons on homonymous α-motoneurons of the spinal cord of the lower leg antagonist muscles.
Method of recording disynaptic reciprocal inhibition of homonymous α-motoneurons of the spinal cord and presynaptic inhibition (D2 inhibition) of homonymous Ia fibers
Conditioning (n. peroneus profundus) and testing stimuli (n. tibialis) were applied to each study subject with an interstimulus interval of 3 ms and 100 ms (Figure 1B). A short-delay conditioning stimulus of 3 ms before the testing stimulus activates the afferents of M. tibialis anterior and excites Ia inhibitory interneurons to the α-motoneurons of soleus, thereby suppressing the testing H-response of soleus and causing functional changes in the activity of reciprocal inhibition at the spinal level [19, 20]. A long-delay conditioning stimulus activates Ia inhibitory interneurons through excitatory Ia interneurons to soleus α-motoneurons 100 ms before the testing stimulus, which, in turn, reduces the excitability of α-motoneurons of the corresponding muscle [2, 20]. The control H-reflex was employed to determine the manifestation of reciprocal and presynaptic inhibition, which was calculated by the formula: Amplitude of testing H-response (mV) / Amplitude of control H-response (mV) × 100. Manifestation of reciprocal and presynaptic inhibition was evaluated by the most pronounced suppression of testing H-reflex (%). The strength of the control and testing stimuli on n. tibialis was 15-25% of the intensity generating maximum amplitude of the soleus H-reflex, and of the conditioning stimulus on n. peroneus profundus was 5-15% of the stimulus magnitude generating maximum amplitude of the tibialis anterior M-response. The amplitudes of H-reflexes and M-responses were recorded on a miniature electromyograph, using the MYO software developed by the Autonomous Nonprofit Organization, Institute of Medical Rehabilitation Vozvrashcheniye (St. Petersburg, Russia, 2003). Conditioning and testing stimulation of Ia afferents was carried out. EMG activity of antagonist muscles (m. soleus, m. tibialis anterior) was recorded by surface cutaneous electrodes with a diameter of 9 mm: the active electrode was attached to the projection of the muscle motor point, the reference electrode was placed at 2 cm of the tendon [3].
The method for recording voluntary muscle contraction
Plantar flexion of the foot (isometric contraction type) was used as a motor model. The retention value of the isometric reduction was 5% of the maximum voluntary contraction (MVC). When performing a weak muscle effort, the subjects were positioned in a supine position, the foot of the right leg was rigidly fixed using adjustable straps on a dynamometer platform (Biodex Multi-Joint System Pro-3, USA, 2006). At the beginning of each experiment, the subjects performed MVC of the lower leg muscles. After that, they were proposed to execute a static force of 5% of the MVC and hold it for 20 minutes. Weak muscle contraction was controlled by the subjects visually on a computer monitor. The choice of a weak MVC was due to the fact that the subjects could maintain such muscle tension during a 20-minute tESCS session.
Experimental design
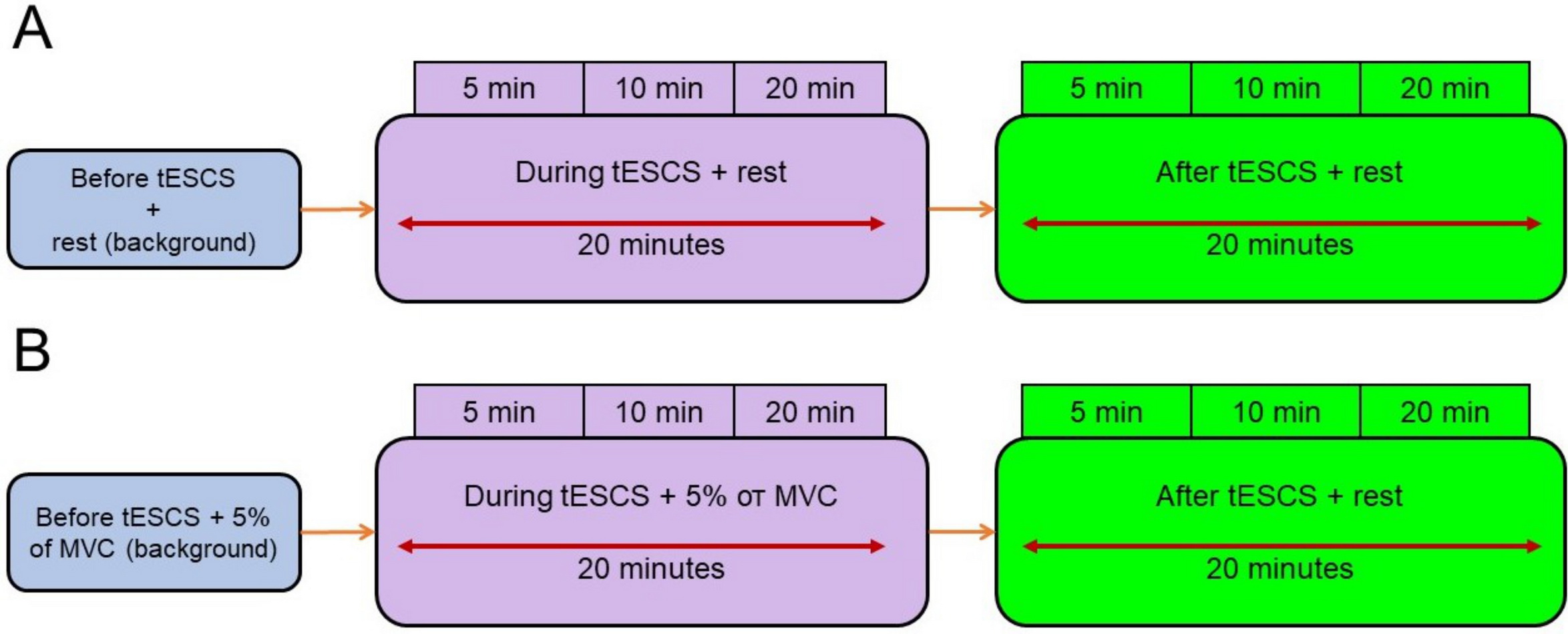
Recordings of the amplitudes of the testing H-reflexes of m. soleus (reciprocal and presynaptic inhibition) were carried out under two experimental conditions. The first experiment involved recordings performed at rest prior to the exposure to prolonged tESCS; then at 5, 10, and 20 minutes of stimulation; and at 5, 10, and 20 minutes of electrical aftereffect (Figure 2A). The second experiment was conducted while holding a weak isometric contraction (5% of MVC) of a muscle to tESCS: during its exposure, at 5, 10, and 20 minutes, while maintaining the force of 5% of MVC; and after stimulation, for 5, 10, and 20 minutes without effort retention of 5% of MVC (Figure 2B). The control H-reflexes of m. soleus were recorded in a state of a relative muscle rest.
Figure 2. Experimental conditions for recording the amplitudes of testing H-reflexes of m. soleus under conditions of short-delay and long-delay conditioning stimulation of n. peroneus profundus and testing stimulation of n. tibialis (disynaptic reciprocal and presynaptic inhibition): A – before (background), during, and after tESCS in a state of a relative muscle rest; B –before (background), during tESCS with force retention of 5% of MVC, and after an exposure to tESCS at rest.
Statistical data processing
Statistical data processing was carried out using Statistica v.12.5.192.7 (StatSoft, USA, 2014). Statistically significant differences in the studied parameters were revealed using the nonparametric Kruskal-Wallis ANOVA method. The normality of the sample distribution was determined using Shapiro-Wilk W test. Multiple comparisons of the testing H-reflex amplitude of m. soleus with the control reflex (%) were conducted during and after tESCS for 5, 10, and 20 minutes, compared with the background values (i.e., at rest) (P5 min tESCS × background; P10 min tESCS × background; P20 min tESCS × background; P5 min after tESCS × background; P10 min after tESCS × background; P20 min after tESCS × background); and also between 5 and 10 (P5 min×10 min tESCS; P5 min×10 min after tESCS), 5 and 20 (P5 min×20 min tESCS; P5 min×20 min after tESCS), and 10 and 20 minutes (P10 min×20 min tESCS; P10 min×20 min after tESCS) during and after stimulation effects. Similar multivariate analysis was conducted with introducing force retention of 5% of MVC. Then the differences in the manifestation of reciprocal inhibition and presynaptic inhibition were determined under different experimental conditions (Kruskal-Wallis ANOVA). The critical value of statistical significance level, when testing null hypotheses, was assumed at P=0.05.
Results
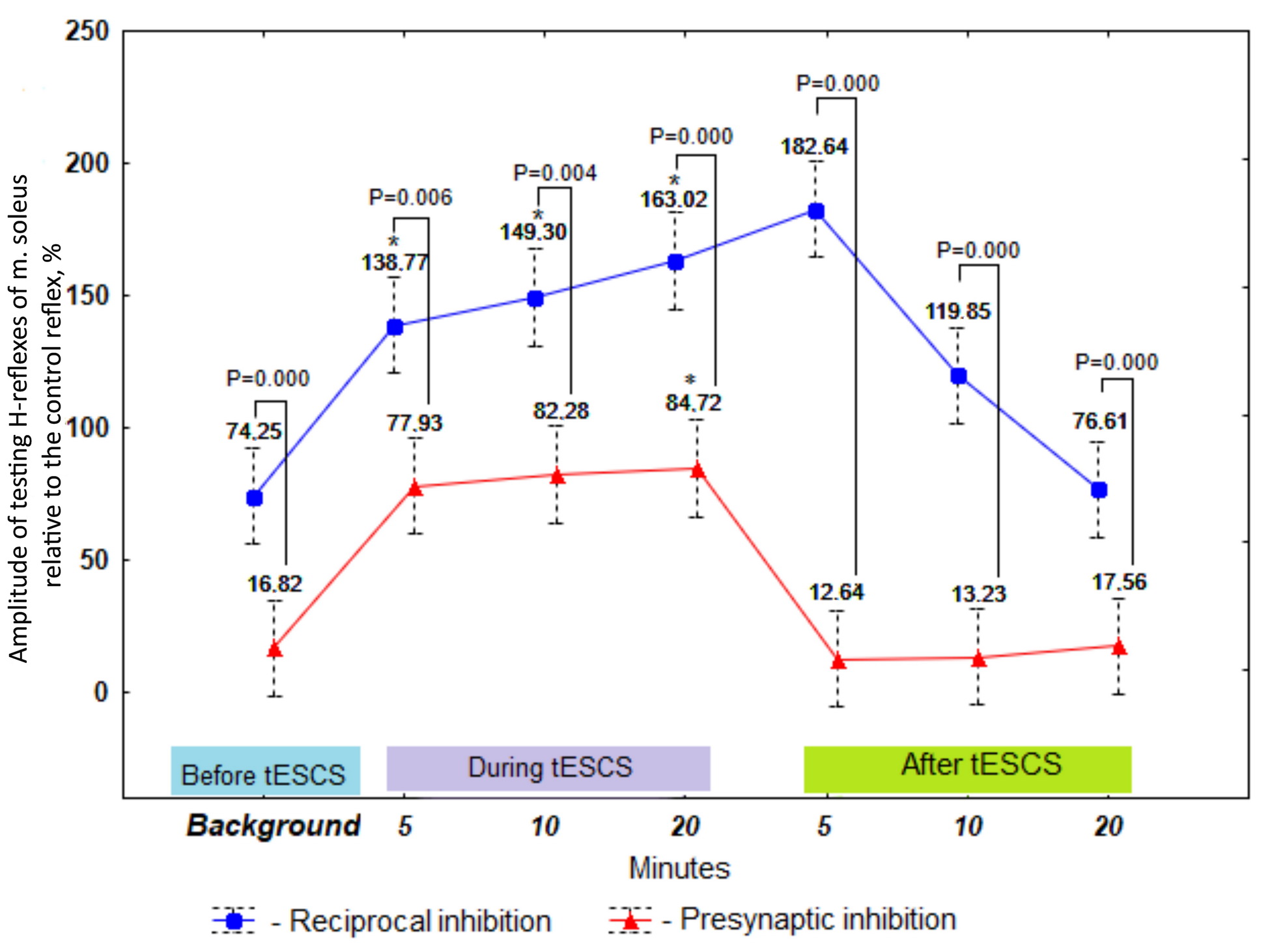
The data in Figure 3 indicate that before, during, and after stimulation of the spinal cord at rest, the manifestation of presynaptic inhibition was greater vs. the manifestation of reciprocal inhibition (P-value ranging from 0.000 to 0.006). Twenty-minute session of tESCS yielded a statistically significant reduction in the activity of reciprocal inhibition, compared with the background rate (P5 min tESCS × background=0.007; P10 min tESCS × background=0.005; P20 min tESCS × background=0.001) and with presynaptic inhibition – given only 20 minutes of stimulation (P20 min tESCS × background=0.037), while reciprocal inhibition was inverted to reflex reciprocal facilitation. Within twenty minutes of stimulating impact on the spinal cord, the manifestation of reciprocal facilitation (P5 min×10 min tESCS=1.000; P5 min×20 min tESCS=1.000; P10 min×20 min tESCS=1.000) and presynaptic inhibition (P5 min×10 min tESCS=1.000; P5 min×20 min tESCS=1.000; P10 min×20 min tESCS=1.000) remained constant. After the end of electrostimulation of the spinal cord, reciprocal facilitation was recorded at 5 (P5 min after tESCS × background=0.612) and 10 (P10 min after tESCS × background=1.000) minutes of aftereffect, and did not differ from the background values. At the twentieth minute of the tESCS aftereffect, reciprocal inhibition was recorded, and it matched the background values (P20 min after the tESCS × background=1.000). It is worth noting that the severity of presynaptic inhibition within 20 minutes after the end of stimulation did not change and was similar to the background values (Figure 3; P5 min after tESCS × background=0.612; P10 min after tESCS × background=1.000; P20 min after tESCS × background=1.000).
Figure 3. The amplitude of testing H-reflexes of m. soleus as a proportion of the control reflex before, during, and after tESCS at rest (%), M±SE, n=10.
* The reliability of differences relative to background values at P<0.05.
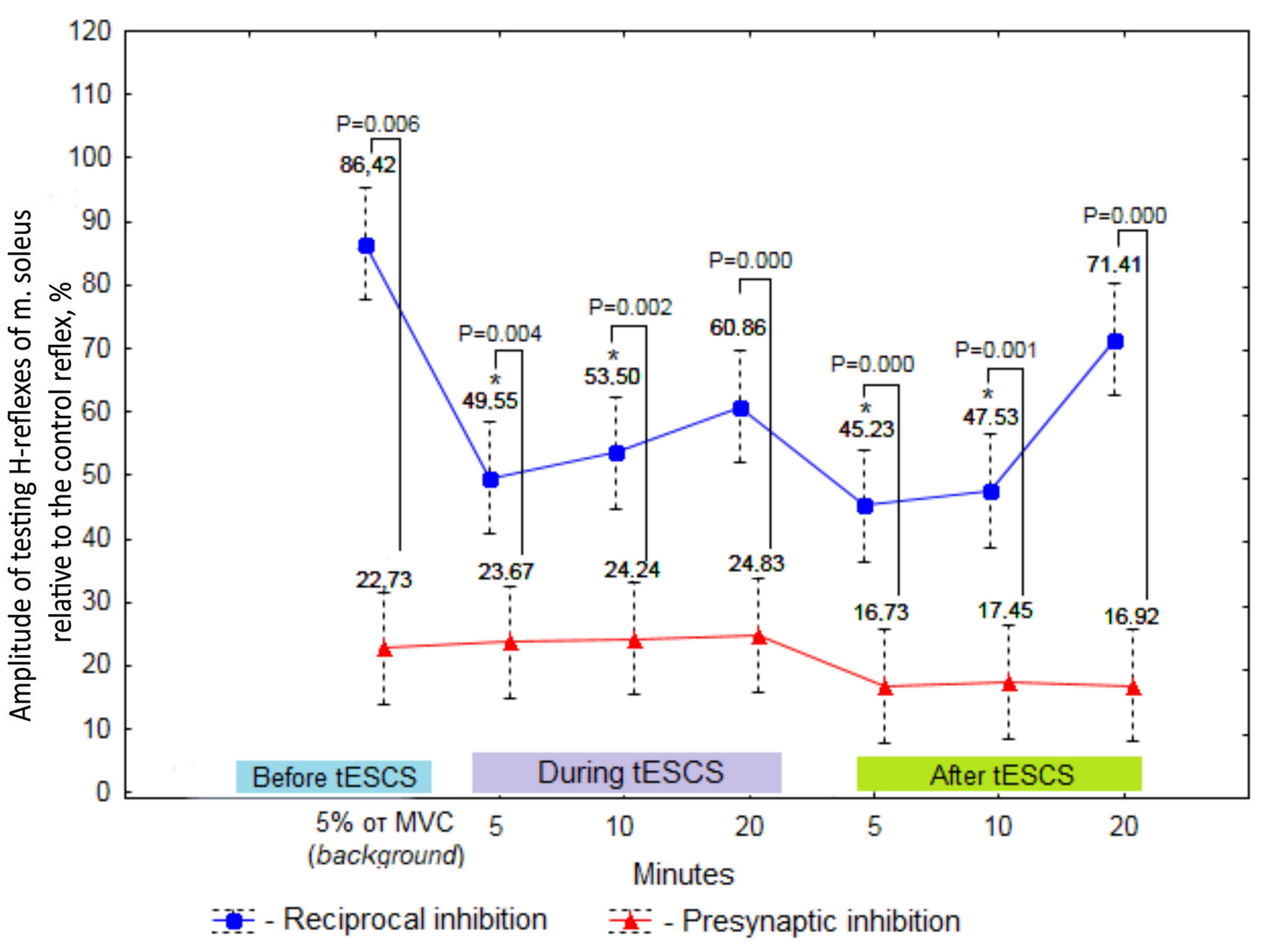
Multivariate analysis of amplitude values of the testing H-reflexes showed that electrical stimulation of the spinal cord in combination with the retention of a weak muscular tension of 5% of MVC yielded an increased reciprocal inhibition at 5 (P5 min tESCS+5% of MVC×5% of MVC (background)=0.010) and 10 (P10 min tESCS+5% of MVC×5% of MVC (background)=0.037) minutes of exposure, compared with the value prior to stimulation via maintaining a weak isometric contraction (Figure 4). Similar effect in manifestation of reciprocal inhibition was observed at the end of stimulation (P5 min after tESCS×5% of MVC (background)=0.002; P10 min after tESCS×5% of MVC (background)=0.005). By the twentieth minute after applying tESCS, the reciprocal inhibition was restored to the background values (P20 minutes after the tESCS×5% of the MVC (background)=1.000). Within twenty minutes of the spinal cord stimulation in combination with isometric force of 5% of MVC, reciprocal inhibition remained constant (P5 min tESCS+5% of MVC×10 min tESCS+5% of MVC=1.000; P5 min tESCS+5% of MVC×20 min tESCS+5% of MVC=1.000; P10 min tESCS+5% of MVC×20 min tESCS+5% of MVC=1.000).
Figure 4. The amplitude of testing H-reflexes of m. soleus as a proportion of the control reflex before tESCS at rest (background), with force retention of 5% of MVC, during tESCS in combination with retention of 5% of MVC, and after an exposure to tESCS at rest (%), M±SE, n=10.
* The reliability of differences relative to background values at P<0.05.
Multivariate analysis of amplitude values of the testing H-reflexes showed that electrical stimulation of the spinal cord in combination with the retention of a weak muscular tension of 5% of MVC yielded an increased reciprocal inhibition at 5 (P5 min tESCS+5% of MVC×5% of MVC (background)=0.010) and 10 (P10 min tESCS+5% of MVC×5% of MVC (background)=0.037) minutes of exposure, compared with the value prior to stimulation via maintaining a weak isometric contraction (Figure 4). Similar effect in manifestation of reciprocal inhibition was observed at the end of stimulation (P5 min after tESCS×5% of MVC (background)=0.002; P10 min after tESCS×5% of MVC (background)=0.005). By the twentieth minute after applying tESCS, the reciprocal inhibition was restored to the background values (P20 minutes after the tESCS×5% of the MVC (background)=1.000). Within twenty minutes of the spinal cord stimulation in combination with isometric force of 5% of MVC, reciprocal inhibition remained constant (P5 min tESCS+5% of MVC×10 min tESCS+5% of MVC=1.000; P5 min tESCS+5% of MVC×20 min tESCS+5% of MVC=1.000; P10 min tESCS+5% of MVC×20 min tESCS+5% of MVC=1.000).
The analysis of presynaptic inhibition manifestation implied that this inhibitory mechanism did not change as during spinal cord stimulation with an isometric force of 5% of MVC (P5 min tESCS+5% of MVC×5% of MVC (background)=1.000; P10 min tESCS+5% of MVC×5% of MVC(background)=1.000; P20 min tESCS+5% of MVC×5% of MVC(background)=1.000), and in the postactivation period (P5 min after tESCS×5% of MVC (background)=1.000; P10 min after tESCS×5% of MVC (background)=1.000; P20 min after tESCS×5% of MVC (background)=1.000).
The data presented in Figure 4 suggest that activity of presynaptic inhibition was expressed to a greater extent during spinal cord stimulation when performing a weak voluntary effort, as well as during the postactivation period (P-values ranging from 0.000 to 0.006).
Discussion
The presented results reflect the contribution of long-term tESCS to neuromodulation of the functional activity of reciprocal and presynaptic inhibition in the system of lower leg antagonist muscles in healthy subjects. The study of inhibitory interneuron circuits at the spinal level, such as disynaptic reciprocal, recurrent, nonreciprocal, and presynaptic inhibition, is crucial for understanding the reflex mechanisms providing sensorimotor functions of human motor control in normal and pathological conditions.
The results of a study of tESCS effect on the functional activity of spinal inhibition in the system of antagonist muscles of the lower leg in humans demonstrated that during 20-minute spinal cord stimulation at rest, the manifestation of reciprocal inhibition decreased, inverting to reciprocal facilitation; while presynaptic inhibition (D2 inhibition) weakened only on the twentieth minute of stimulation. In postactivation period, the activity of spinal inhibition processes in a system of antagonist muscles was consistent with background levels (Figure 2). During and after the end of the spinal cord stimulation at rest, the most pronounced manifestation of presynaptic inhibition was revealed, compared with reciprocal inhibition. Contradictory results were obtained in the study by T. Yamaguchi et al. [21], who examined the postactivation effects of a 15-minute continuous current (stimulation strength of 2 mA) at rest on the manifestation of short-delay presynaptic (D1-inhibition) and reciprocal inhibitory interaction in the antagonist muscle system of healthy individuals. The authors discovered that after electrical stimulation of the spinal cord, presynaptic inhibition weakened within 15 minutes of aftereffect, whereas reciprocal inhibition did not differ from the initial level (background). Nevertheless, the results of the same authors from their use of long-term tESCS with a pulse repetition frequency of 100 Hz showed that after 20 minutes of the spinal cord electrical stimulation, reciprocal inhibition increased within 15 minutes of aftereffect, and presynaptic inhibition was more pronounced than reciprocal inhibition and did not differ from the baseline level for 30 minutes of the aftereffect [22]. Scientists suggested that long-term electrical stimulation of the spinal cord may have induced short-term plastic changes in the Ia interneurons of reciprocal inhibition.
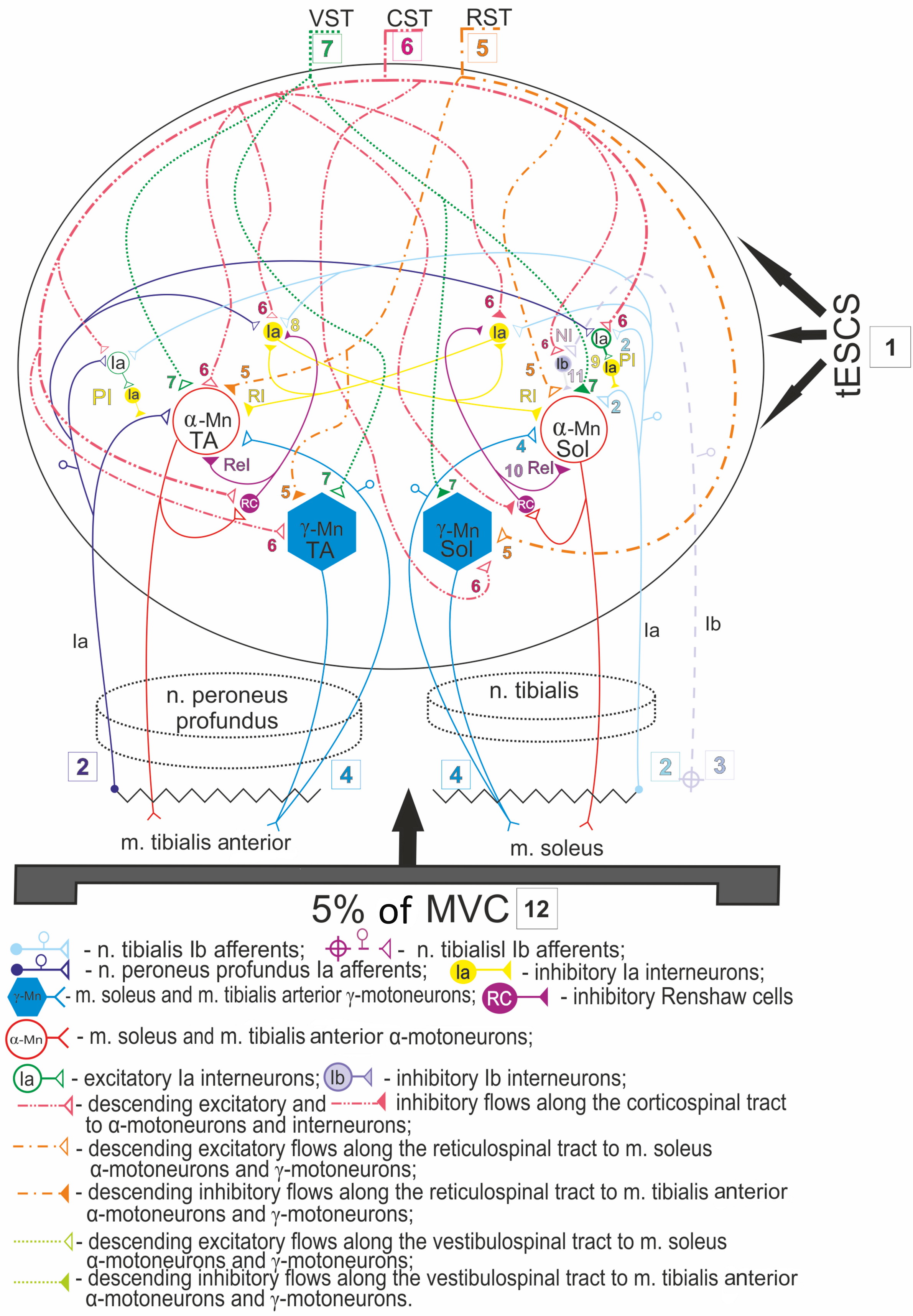
It is known that applying tESCS involves afferents of groups Ia and Ib, afferents of group II, excitatory and inhibitory spinal interneurons that implement poly- and oligosynaptic reflexes, as well as pyramidal and extrapyramidal tracts [8, 9]. Based on the opinion of these authors, we believe that during 20-minute exposure to tESCS (1) at rest, ascending peripheral influences from Ia (2) and Ib (3) afferents and efferent effects of γ-motoneurons on α-motoneurons (4), as well as descending excitatory and inhibitory supraspinal influences of reticulospinal (5) and vestibulospinal (7) tracts on corresponding motoneurons are consistently involved, which leads to an increase in reciprocal facilitation and decrease in the effect of presynaptic inhibition on the motoneuron pool of the foot flexor muscle (m. soleus) (Figure 5).
Figure 5. The proposed model of inhibitory interneuron circuit of lower leg antagonist muscles mediated by ascending and descending influences on spinal motoneurons during and after 20-minute transcutaneous electrical spinal cord stimulation (tESCS) in combination with weak muscle tension.
1 – transcutaneous electrical spinal cord stimulation (tESCS); 2, 3, 4 – ascending influences from peripheral afferents and afferents of γ-motoneurons; 5, 6, 7 – supraspinal descending influences from the reticulospinal, corticospinal, and vestibulospinal tracts; 8 – reciprocal inhibition via Ia inhibitory interneuron; 9 – presynaptic inhibition via primary excitatory Ia interneuron to the secondary Ia inhibitory interneuron; 10 – recurrent inhibition via Renshaw cell; 11 – nonreciprocal (Ib) inhibition via inhibitory Ib interneuron; 12 – 5% of MVC (maximum voluntary contraction); RST, reticulospinal tract; CST, corticospinal tract; VST, vestibulospinal tract; PI, presynaptic inhibition. RI, reciprocal inhibition; ReI, recurrent inhibition; NI, nonreciprocal inhibition.
The most pronounced effect of a long-term tESCS was observed when performing a weak isometric contraction, which was accompanied by an increase in reciprocal inhibition activity during 10 minutes of exposure and 10 minutes after the end of stimulation. The severity of presynaptic inhibition during and after the spinal cord stimulation remained unchanged and was similar to the background values. The presynaptic inhibition activity was expressed to a greater extent during the long-term stimulation of the spinal cord at rest and when performing a weak voluntary effort, as well as during the postactivation period (Figure 4). It is believed that when maintaining a moderate static effort, presynaptic inhibition regulates excessive afferent influx to the α-motoneurons of the agonist and antagonist muscles of the lower leg, disinhibiting nonreciprocal and reciprocal inhibitory effects on them, thereby ensuring normal human motor activity [7]. It is likely that the same reflex mechanism can be mediated by the effects of tESCS.
In a series of studies by several authors, it was shown experimentally that the corticospinal tract forms poly- and oligosynaptic connections with inhibitory interneurons of groups Ia and Ib, and Renshaw cells; and performs coordinated downward effects of motor input parameters on the motor centers of both homonymous and heteronymous α-motoneurons through inhibitory interneuronal mechanisms (presynaptic, reciprocal, nonreciprocal, and recurrent inhibition) [3, 2, 23]. The descending lateral and ventral corticospinal pathways are glutamatergic, exercising mono- and polysynaptically excitatory effects on α-motoneurons, along with polysynaptically excitatory effects on γ-motoneurons [24]. However, lateral vestibulospinal pathways exert polysynaptic facilitating effects on α- and γ-motoneurons of extensor muscles, and inhibitory effects on α- and γ-motoneurons of flexor muscles of the lower and upper limbs. At the same time, the lateral reticulospinal pathways have polysynaptic effects that are opposite to the vestibulospinal tract, exerting an inhibitory effect on α- and γ-motoneurons of extensor muscles and excitatory effects on motoneurons of flexor muscles. There is also an opinion that the rubrospinal tract exerts excitatory effects on the motor centers of skeletal muscles [25].
Recent data using transcranial magnetic stimulation (TMS) of cortical structures of the brain imply that Ia interneurons of reciprocal and presynaptic inhibition act as a common end pathway and can be modulated via corticospinal, reticulospinal and vestibulospinal connections of the spinal cord [23, 26, 27]. Activation of the corticospinal pathways by TMS revealed weakening of reciprocal and presynaptic inhibition of the Ia afferents of the lower limb muscles in a subject at rest [28, 23]; while activation of reticulospinal and vestibulospinal pathways in humans increased the severity of presynaptic inhibition, and reciprocal inhibition, on the contrary, decreases the severity [26]. As we pointed out earlier, supraspinal (reticulospinal and vestibulospinal pathways) descending influences caused by nonspecific activation of brainstem structures (Jendrassik maneuver) also modulate the activity of presynaptic inhibition of Ia afferents of the foot flexor muscle, depending on the type (concentric, eccentric, isometric) and strength (50% and 100% MVC) of muscle contraction [29].
Using our data, we developed a scheme of putative reflex mechanisms mediated by descending supraspinal and ascending peripheral influences on inhibitory interneuron circuits of the spinal cord in the system of antagonist muscles of the lower leg in humans based on tESCS effects (Figure 5). The use of a 20-minute tESCS (1) in combination with a weak isometric contraction (12) and its postactivation effect, apparently, manifests itself in additional activation of excitatory corticospinal (6) and peripheral influences from Ia afferents (2) on Ia inhibitory interneurons of reciprocal inhibition (8) and Ia interneurons of presynaptic inhibition (9), thereby increasing their functional activity. Presumably, the descending vestibulospinal (7) and reticulospinal (5) pathways, in addition to the corticospinal (6), exert excitatory and inhibitory effects on the motoneuron pool of antagonist muscles (m. soleus and m. tibialis anterior), providing coordinated work of all spinal inhibitory systems. It should also be taken into account that the mechanisms of reciprocal inhibition (10), mediated by Renshaw cells, which have an inhibitory effect on Ia interneurons of reciprocal inhibition (8) and Ib interneurons of nonreciprocal inhibition (9), are also involved in the regulation of reciprocal inhibition [2, 3]. Our data indicate that the activity of Renshaw cells in recurrent inhibition is more pronounced upon stimulation of the spinal cord in combination with a weak voluntary effort, compared with nonreciprocal inhibition [12]. Yet, the postactivation effect was manifested by similar changes in the severity of recurrent and nonreciprocal inhibition: an increase within 10 minutes, followed by a decrease to background values 20 minutes after the end of stimulation.
In recent decades, robotic mechanotherapy and tESCS have become widespread in rehabilitation of patients with movement disorders caused by diseases and injuries of the spinal cord [14, 16, 17, 30-32]. Several studies demonstrated an effective influence of locomotor training on improving the functional activity of pre- and postsynaptic inhibitory systems of the spinal cord [30-32]. The restoration of the processes of presynaptic and postsynaptic inhibition in patients with spinal cord injuries after locomotor training and course exposure to tESCS is based on the mechanism of neuroplasticity, i.e., on capability of the nervous tissue to structural and functional reorganization that occurs after its damage, while modulation (excitation, or inhibition, or harmonization) occurs in the neural circuits of the spinal cord [18, 33]. At the same time, these data imply an importance of spinal inhibitory interneuron circuits in the partial or complete restoration of motor function in movement disorders and spinal cord injuries. Hence, tESCS could be one of the ways to restore the functional activity of inhibitory interneuron circuits of the spinal cord in rehabilitation of patients with movement disorders caused by spinal cord diseases and injuries.
Conclusion
Our data filled the gap in scientific knowledge on the mechanisms of functioning of spinal inhibitory systems of lower leg antagonist muscles in healthy subjects under the influence of transcutaneous electrical stimulation of the spinal cord. Long-term electrical stimulation of the spinal cord neuromodulates reciprocal and presynaptic inhibition of spinal α-motoneurons in the state of a relative muscle rest and with retention of weak muscle tension. The effect of tESCS at rest leads to diminished functional activity of the inhibitory spinal neural structures of antagonist muscles; but when maintaining a weak muscle tension, on the contrary, to their strengthening; moreover, presynaptic inhibition is the most pronounced. It is possible that revealed multidirectional changes in the activity of spinal inhibitory mechanisms in the antagonist muscle system, based on the effects of prolonged electrical stimulation of the spinal cord, are associated with the fact that when voluntary muscle tension is performed, inhibitory interneuronal circuits of the spinal cord are affected by a wider range of descending supraspinal and ascending peripheral influences, compared with spinal cord stimulation in the state of a relative muscle rest. The fundamental data obtained as a result of such research could find practical application in rehabilitation of patients with movement disorders caused by spinal cord diseases and injuries.
Conflict of interest
The authors declare the absence of apparent and potential conflicts of interest associated with the publication of this article.
Ethical approval
All the subjects were informed about the course of the experiment and gave written informed consent to participate in the study. The study was conducted in compliance with the Declaration of Helsinki of the World Medical Association and approved by the Ethics Committee at Velikiye Luki State Academy of Physical Culture and Sports.
- Bikmyllina RKh, Rozental' AN, Pleshchinskiĭ IN. Inhibitory systems of the spinal cord in the control of interactions of functionally coupled muscles. Fiziol Cheloveka 2007; 33(1): 119-130. Russian. https://pubmed.ncbi.nlm.nih.gov/17361617.
- Pierrot-Deseilligny E, Burke DC. The circuitry of the human spinal cord: Spinal and corticospinal mechanisms of movement. Cambridge: Cambridge University Press, 2012; xxiii, 606 p. https://www.worldcat.org/title/circuitry-of-the-human-spinal-cord-spinal-and-corticospinal-mechanisms-of-movement/oclc/1105473253.
- Chelnokov AA, Gorodnichev RM. Patterns of Spinal Inhibition Formation in Humans. Moscow: INFRA-M Academic Publishing LLC, 2021: 192 p. Russian. https://doi.org/10.12737/1039428.
- Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res 1999; 129(1): 1-37. https://doi.org/10.1007/s002210050933.
- Earles DR, Dierking JT, Robertson CT, Koceja DM. Pre- and post-synaptic control of motoneuron excitability in athletes. Med Sci Sports Exerc 2002; 34(11): 1766-1772. https://doi.org/10.1097/00005768-200211000-00012.
- Kitano K, Tsuruike M, Robertson CT, Kocejal DM. Effects of a complex balance task on soleus H-reflex and presynaptic inhibition in humans. Electromyogr Clin Neurophysiol 2009; 49(5): 235-243. https://pubmed.ncbi.nlm.nih.gov/19694211.
- Chelnokov AA, Buchatskaya IN. Functional features spinal inhibition during voluntary motor activity. Theory and Practice of Physical Culture 2015; (6): 4. https://www.elibrary.ru/item.asp?id=25825195.
- Gerasimenko Y, Gorodnichev R, Machueva E, Pivovarova E, Semyenov D, Savochin A, et al. Novel and direct access to the human locomotor spinal circuitry. J Neurosci 2010; 30(10): 3700-3708. https://doi.org/10.1523/JNEUROSCI.4751-09.2010.
- Gorodnichev R, Pivovarova EA, Puhov A, Moiseev SA, Savochin A, Moshonkina TR, et al. Transcutaneous electrical stimulation of the spinal cord: A noninvasive tool for activating stepping pattern generators in humans. Hum Physiol 2012; 38: 158-167. Russian. https://doi.org/10.1134/S0362119712020065.
- Yapharova GG, Militskova AD, Shulman AA, Spiridonova KN, Bikchentaeva LM. The effect of transcranial magnetic stimulation on the responses of the leg muscles caused by percutaneous electric stimulation of the spinal cord. Practical Medicine 2017; (8 (109)): 201-205. Russian. https://www.elibrary.ru/item.asp?id=30060651.
- Roshchina LV, Chelnokov AA. Effect of percutaneous electrical stimulation of spinal cord on human motor system functionality. Theory and Practice of Physical Culture 2020; (4): 30. Russian. https://www.elibrary.ru/item.asp?id=42667417.
- Chelnokov AA, Roshchina LV, Gladchenko DA, Pivovarova EA, Piskunov IV, Gorodnichev RM. Effect of transcutaneous electrical stimulation of the spinal cord on the functional activity of spinal inhibition in the system of lower leg synergist muscles in humans. Human Physiology 2022; 48(2): 14-27. Russian. https://doi.org/10.31857/S0131164622020035.
- Roshchina LV, Gladchenko DA, Pivovarova EA, Chelnokov AA. Effect of long-term electrical spinal cord stimulation on expression of non-reciprocal inhibition of α-motoneurons in human skeletal muscles. RUDN Journal of Medicine 2019; 23(4): 390-396. Russian. https://doi.org/10.22363/2313-0245-2019-23-4-390-396.
- Ikoeva GA, Nikityuk IE, Kivoenko OI, Moshonkina TR, Solopova IA, Sukhotina IA, et al. Clinical, neurological, and neurophysiological evaluation of the efficiency of motor rehabilitation in children with cerebral palsy using robotic mechanotherapy and transcutaneous electrical stimulation of the spinal cord. Pediatric Traumatology, Orthopaedics and Reconstructive Surgery 2016; 4(4): 47-55. Russian. https://doi.org/10.17816/PTORS4447-55.
- Savenkova AA, Sarana AM, Shcherbak SG, Gerasimenko YuP, Moshonkina TR. Noninvasive spinal cord electrical stimulation in the complex rehabilitation of patients with spinal cord injury. Problems of Balneology, Physiotherapy, and Exercise Therapy 2019; 96(5): 11-18. Russian. https://doi.org/10.17116/kurort20199605111.
- Moshonkina TR, Pogolskaya MA, Vinogradskaya ZV, Likhacheva PK, Gerasimenko YuP. Transcutaneous spinal cord electrical stimulation in motor rehabilitation of patients with spinal cord injury. Integrative Physiology 2020; 1(4): 351-365. Russian. https://doi.org/10.33910/2687-1270-2020-1-4-351-365.
- Islam MA, Pulverenti TS, Knikou M. Neuronal actions of transspinal stimulation on locomotor networks and reflex excitability during walking in humans with and without spinal cord injury. Front Hum Neurosci 2021; 15: 620414. https://doi.org/10.3389/fnhum.2021.620414.
- Smit AC, Knikou MA. A review on locomotor training after spinal cord injury: Reorganization of spinal neuronal circuits and recovery of motor function. Neural Plast 2016; 2016: 1216258. https://doi.org/10.1155/2016/1216258.
- Crone C, Hultborn H, Jespersen B, Nielsen J. Reciprocal Ia inhibition between ankle flexors and extensors in man. J Physiol 1987; 389: 163-185. https://doi.org/10.1113/jphysiol.1987.sp016652.
- Chelnokov AA, Gorodnichev RM. Age-related features in the formation of spinal inhibition of skeletal muscles in males. Hum Physiol 2015; 41: 644-651 Russian. https://doi.org/10.1134/S036211971506002X.
- Yamaguchi T, Fujimoto S, Otaka Y, Tanaka S. Effects of transcutaneous spinal DC stimulation on plasticity of the spinal circuits and corticospinal tracts in humans. In: 6th International IEEE/EMBS Conference on Neural Engineering (NER). San Diego, USA: IEEE 2013: 275-278. https://doi.org/10.1109/NER.2013.6695925.
- Yamaguchi T, Fujiwara T, Takahara T, Takahashi Y, Mizuno K, Ushiba J, et al. The effects of transcutaneous spinal cord stimulation on spinal reciprocal inhibition in healthy persons. Clinical Neurophysiology 2017; 128(3): e115-e116. https://doi.org/10.1016/j.clinph.2016.10.326.
- Kubota S, Uehara K, Morishita T, Hirano M, Funase K. Inter-individual variation in reciprocal Ia inhibition is dependent on the descending volleys delivered from corticospinal neurons to Ia interneurons. J Electromyogr Kinesiol 2014; 24(1): 46-51. https://doi.org/10.1016/j.jelekin.2013.11.004.
- Korolev AA. Functional anatomy of the descending motor systems in normal and formation spastic paresis. Fundamental Research 2013; (3-1): 92-96. Russian. https://www.elibrary.ru/item.asp?id=18809092.
- Fujito Y, Aoki M. Monosynaptic rubrospinal projections to distal forelimb motoneurons in the cat. Exp Brain Res 1995; 105(2): 181-190. https://doi.org/10.1007/BF00240954.
- Matsugi A, Mori N, Uehara S, Kamata N, Oku K, Okada Y, et al. Effect of cerebellar transcranial magnetic stimulation on soleus Ia presynaptic and reciprocal inhibition. Neuroreport 2015; 26(3): 139-143. https://doi.org/10.1097/WNR.0000000000000315.
- Lopez AJ, Xu J, Hoque MM, McMullen C, Kesar TM, Borich MR. Integration of convergent sensorimotor inputs within spinal reflex circuits in healthy adults. Front Hum Neurosci 2020; 14: 592013. https://doi.org/10.3389/fnhum.2020.592013.
- Meunier S. Modulation by corticospinal volleys of presynaptic inhibition to Ia afferents in man. J Physiol Paris 1999; 93(4): 387-394. https://doi.org/10.1016/s0928-4257(00)80066-2.
- Bogdanov S, Gladchenko D, Roshchina L, Chelnokov A. Effect of supraspinal influences on the manifestation of presynaptic inhibition Ia afferents in different types of muscle contraction in humans. RUDN Journal of Medicine 2020; 24(4): 338-344. Russian. https://doi.org/10.22363/2313-0245-2020-24-4-338-344.
- Mummidisetty CK, Smith AC, Knikou M. Modulation of reciprocal and presynaptic inhibition during robotic-assisted stepping in humans. Clin Neurophysiol 2013; 124(3): 557-564. https://doi.org/10.1016/j.clinph.2012.09.007.
- Knikou M, Mummidisetty CK. Locomotor training improves premotoneuronal control after chronic spinal cord injury. J Neurophysiol 2014; 111(11): 2264-2275. https://doi.org/10.1152/jn.00871.2013.
- Knikou M, Smith AC, Mummidisetty CK. Locomotor training improves reciprocal and nonreciprocal inhibitory control of soleus motoneurons in human spinal cord injury. J Neurophysiol 2015; 113(7): 2447-2460. https://doi.org/10.1152/jn.00872.2014.
- Knikou M. Plasticity of corticospinal neural control after locomotor training in human spinal cord injury. Neural Plast 2012; 2012: 254948. https://doi.org/10.1155/2012/254948.
Received 22 December 2021, Revised 22 February 2022, Accepted 6 June 2022
© 2021, Russian Open Medical Journal
Correspondence to Denis A. Gladchenko. Address: Velikiye Luki State Academy of Physical Culture and Sports, 4 Yubileynaya Square, Velikiye Luki 182105, Russia. E-mail: gladchenko84@outlook.com.