Introduction
Chronic obstructive pulmonary disease (COPD) kills 3.2 million people annually worldwide [1]. COPD is characterized by inflammation of the lung parenchyma and pulmonary hypoxemia with a confirmed systemic component. In COPD, a chronic inflammatory process in the respiratory tract contributes to the systemic inflammation and is accompanied by an increase in the level of circulating cytokines (tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-8, IL-18), chemokines, acute phase proteins, and white blood cells [2]. Systemic inflammation is a pathogenetic mechanism that underlies most of the systemic manifestations of respiratory tract diseases. Despite the fact that the clinical signs of chronic systemic inflammation in COPD are well known, the molecular mechanisms for regulating the inflammatory response remain unknown.
Inflammatory responses are regulated by a wide range of cells and signaling molecules. The main participants in the induction and resolution of inflammation in many inflammatory diseases, including COPD, are leukocytes and cytokines, as well as lipid mediators [3-4]. Signaling lipids such as eicosanoids, phosphoinositides, sphingolipids, and fatty acids (FAs) control most cellular processes, including cell proliferation, apoptosis, metabolism, and migration. FAs and their oxidized derivatives play an important role in the immune cell activities and cellular signaling [3, 5-7]. FAs affect the fundamental properties of the cell membrane of immune cells, including its fluidity, elasticity, receptor expression activity, functionality of embedded proteins, and signal transmission through lipid rafts, which leads to changes in cell signaling and modification of gene expression [6]. Chain length, odd carbon content, and saturation vs. unsaturation of FAs have a strong influence on the physical and chemical properties of cell membranes [7].
FAs serve as ligands for immune cell receptors, such as peroxisome proliferator-activated receptor gamma (PPARγ) and G-protein coupled receptor 120 (GPR120), directly affecting the activation status and protein synthesis [8]. The FA incorporation into the plasma membrane of immune cells can alter the synthesis of cytokines. For example, it was shown that after treating the T cell culture with the polyunsaturated fatty acids (PUFAs), the synthesis rate of some cytokines (TNFα, IL-6, IL8, IL1β, IL-2, IL10, and interferon (IFN-γ) increased [9].
The n-3 PUFAs and n-6 PUFAs of phospholipids in cell membranes of immune cells are the main substrates for synthesizing the inflammatory and pro-resolving lipid mediators [3, 10]. Arachidonic acid (20:4n6), the main representative of n-6 PUFAs, is used to synthesize proinflammatory eicosanoids both by the lipoxygenase pathway (leukotriene) and cyclooxygenase pathway (thromboxane, prostaglandin), whereas eicosapentaenoic acid (20:5n3) from the n-3 PUFAs family is a substrate of pro-resolving lipid mediators (maresins, lipoxins, resolvins, protectins) and key participant in the resolution of inflammation [7]. The balance between n-3 PUFAs and n-6 PUFAs determines the path of inflammatory response. The prevalence of n-6 PUFAs and n-3 PUFA deficiency may contribute to impaired resolution of inflammation [10]. Thus, a resolution disorder is considered a significant mechanism for COPD exacerbation and progression. Therefore, the FA composition of the immune cell membrane can directly affect the immune and inflammatory responses.
The study of lipidome in COPD is of great importance. To date, research emphasizes the study of phospholipids, individual PUFAs and their oxidized derivatives in red blood cells, plasma and serum [10-12]. Van der Does A.M. et al. analyzed the composition of PUFAs in sputum and bronchial epithelial cells [13]. In the modern literature, there are no studies of the FA profile of immune cells in COPD. Most scientists focused on the effects of alimentary n-3 PUFAs on clinical outcomes in lung diseases. At the same time, saturated fatty acids (SFAs) are fundamental components of cell membranes. They play a crucial role in cellular functions, including energy metabolism, signal transmission, and the bilayer structure of the membrane [14]. Most SFAs are catabolized by mitochondrial beta-oxidation, providing acetyl-CoA as a substrate for the tricarboxylic acid cycle. Through FA oxidation, adenosine triphosphate (ATP) is produced; however, this is often accompanied by oxidative stress. There is a direct relationship between the impaired SFA metabolism and dysfunction of cell mitochondria, which further contributes to the disruption of energy processes, increased oxidative stress and the development of apoptosis [11].
Any modification of lipid composition and metabolism changes the quality of the plasma membrane and its properties. Despite obvious high significance of changes in the lipidome of immune cells in developing many pathological ailments, an importance of modification of the FA composition in the leukocyte membrane in the developing systemic inflammation in COPD progression is not well understood.
The goal of our study was to investigate the modification of the FA composition of leukocyte membranes in patients with COPD of various severity and to establish a relationship between the FA leukocyte membrane composition and the immune system during the progression of COPD.
Material and Methods
Clinical groups
The study involved 137 patients with COPD. Of them, 50 patients were diagnosed with a mild COPD, 63 patients with a moderate COPD, and 24 patients (18%) with severe COPD. The COPD was diagnosed in compliance with the Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD-2017) [1]. The study was conducted in accordance with the Declaration of Helsinki (2013) and was approved by the Ethics Committee of the Institute of Medical Climatology and Rehabilitation Therapy. All participants signed the written informed consent.
The exclusion criteria from the survey were as follows: any exacerbation at the time of the first visit or during the preceding three months; acute respiratory infections in preceding four weeks; other respiratory diseases; hereditary hyperlipidemia; type 1 and type 2 diabetes; chronic somatic diseases in exacerbation or decompensation; intake of dietary supplements containing PUFAs during the preceding three months. The patients were also excluded if they were not taking any medications, including any cholesterol-lowering medications, and did not use any bronchodilators in the past 24 hours prior to blood sample collection.
The control group consisted of 32 healthy subjects: non-smokers, with normal state of their pulmonary function.
Questionnaire and patient examination
Patients with COPD underwent the following procedures during a single visit: spirometry, testing on the dyspnea scale – mMRC (Modified British Medical Research Council), CAT quality of life assessment (COPD Assessment Test), blood sampling to isolate white blood cells, identification of immunological and cytokine profiles, and the test for content of eicosanoids.
The maximum expiratory volume was measured three times on the Master Screen Body apparatus (CareFusion, Germany) with the highest FEV1 (forced expiratory volume per 1 second) and FVC (forced vital capacity) recorded. The readings were repeated 20 minutes after inhaling 200 μg of salbutamol through a spacer device.
Methods of laboratory testing
Blood samples were collected and further stored in Vacutainer tubes containing EDTA (BD Biosciences) for preparation of peripheral blood mononuclear cells (PBMC) and whole blood stimulation. The peripheral blood leukocytes were isolated via the Ficoll-Verographin density gradient separation. Leukocyte membranes were obtained by lysis of cells in an isolation medium (sucrose 0.75M + EDTA 5 *10-5M + BSA0.5% + 0.01M phosphate buffer). To separate the serum, the blood was collected in 10 mL tubes and centrifuged for 15 minutes at 2,500 rpm; then the serum was taken and frozen at -80 °C until thawed once, and analyzed in batches.
Immunological methods
Immunological examination involved cytofluorimetric determination of subpopulations of immune cells in whole blood, including T lymphocytes (CD3+), T helper cells (CD4), cytotoxic T lymphocytes (CD8), natural killer cells (NK CD16+ 56+), and B cells (CD19+), and measuring serum cytokine levels (TNF-α), interleukins (IL-4, IL-6, IL-10, IL-17A), interferon gamma (IFN-γ) on a BD FACS flow cytofluorimeter using Becton Dickinson kits (USA). Data processing was performed using the FCAP Array 3.0 software. An immunoregulatory index (CD4/CD8) was calculated.
Lipids were extracted from the membranes of the leukocytes using the solvent system of chloroform – methanol, 1:2 (v/v); and then chloroform methanol (1:1 v/v) and 0.9% sodium chloride were added until a complete phase separation was achieved. Methyl ethers of FA were extracted with hexane and purified by micron thin-layer chromatography in benzol. Fatty acid methyl esters were redissolved in hexane and analyzed on a Shimadzu GC-2010 gas-liquid chromatograph (Japan) equipped with a flame ionization detector and a capillary column (0.25 mm × 30 m) with a Supelcowax 10 bonded phase. Column temperature was 210 °C, detector temperature was 250 °C. Helium was used as the carrier gas. FAs were identified by relative retention times and calculated values of the equivalent chain length. We isolate a total of 39 species of saturated (12:0, 14:0, 16:0, 18:0, 20:0), monoenoic (16:1n-9, 16:1n-7, 18:1n-9, 18:1n -7), and polyunsaturated (18:2n-6, 20:3n-6, 20:4n-6, 20:5n-3, 22:4n-6, 22:5n-3, 22:6n-3) FAs of normal structure and isostructure, with a chain length from C12 to C24, with both even and an odd numbers of carbon atoms. The tables do not include individual representatives of the FAs, the content of which did not exceed 0.1%. Basically, these are SFAs of a normal structure (10:0, 19:0, 22:0), some MUFAs (14:1, 18:1n5, 20:1, 22:1), PUFAs (18:2n5/9, 20:3n3) and some iso- and anteiso-fatty acids. The results were expressed as a percentage of the total content of FAs.
The content of eicosanoids in blood serum was assessed by the levels of their stable metabolites – thromboxane B2 (TXB2) and leukotriene B4 (LTB4). Minicolumns (MiniColumns for Sample Preparation, USA) were used to isolate eicosanoids. The quantitative level was determined by an immune-enzyme method with the help of Biotrak EIA system (Amersham Biosciences, UK). Measurements were performed in a 96-well flat bottom microtiter plate on a spectrophotometer (BioTek PowerWave, USA).
The functional state and energy metabolism of leukocytes were assessed by the membrane potential of their mitochondria. Mitochondrial membrane potential (MMP) in leukocytes was measured ex tempore using 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl-carbocyanine iodide (JC-1; MitoProbe™ JC-1 Assay Kit, Life Technologies, USA). JC-1 is a cationic dye that is known to accumulate in mitochondria, forming red aggregates, while the monomeric (green) form remains in the cytoplasm. The ratio of red and green fluorescence was used to determine the MMP. The MMP was studied on a BD FACS CantoII™ flow cytofluorometer. The MMP was assessed as a percentage of cells with reduced MMP.
Statistical analyses
Statistical data processing was performed using Statistica 6.1 software (StatSoft, USA). The hypothesis of normal distribution of quantitative traits in groups was tested using Kolmogorov-Smirnov, Shapiro-Wilk and Pearson’s χ² criteria. As a result, we established that all our data complied with a normal distribution; hence, the data are expressed as M±SD (mean ± standard deviation). The statistical significance of differences between alternative quantitative parameters was assessed using Student’s t-test. To solve the problem of multiple comparisons, the Bonferroni correction was used. The analysis of interrelations between pairs of parameters was carried out using the Spearman’s correlation coefficient (r). Statistically significant differences were assumed at P<0.05.
Results
Clinical, physiological and immune characteristics of patients
Table 1 presents clinical, physiological and immune parameters of the study participants. As can be seen from presented data, the state of the immune system in patients with COPD was characterized by a drop in the absolute number of lymphocytes, CD3+, CD4+, CD8+ (only for patients with mild COPD). Impaired differentiation of immunocompetent cells can reduce the adequate response to the pathogen and development of chronic inflammation.
Table 1. Clinical, physiological and immunological parameters of study subjects
|
Parameters |
Control group (healthy) n=32 |
Mild COPD n=50 |
Moderate CОPD n=63 |
Severe CОPD n=24 |
|
Age, yrs. |
52.3±3.4 |
56.4±4.3 |
57.5±2.5 |
62.4±2.6 |
|
Gender, M/F |
24/8 |
35/15 |
54/9 |
20/4 |
|
Smoking status, % |
|
|
|
|
|
Never smoked |
100 |
32 |
33.3 |
25 |
|
Ex-smokers |
- |
24 |
15.9 |
29.2 |
|
Current smokers |
- |
44 |
50.8 |
25.8 |
|
Pack-years in smokers |
- |
24.8±1.39 |
22.9±1.07 |
23.4±1.34 |
|
Post-bronchodilator test (FEV1), % vs. norm |
101.88 ± 3.23 |
90.13±1.99 |
73.9±2.56 |
48.6±1.76 |
|
Airflow obstruction scale (mMRС), score |
0 |
1 |
2 |
2 |
|
Test САТ, score |
0 |
4-6 |
7-9 |
10-25 |
|
Leukocytes, |
5.51±0.91 |
5.69±0.21 |
5.91±0.11 |
6.51±0.17 (р=0.026)2 (р=0.005)3 |
|
Lymphocytes, % |
34.9±0.9 |
27.3±0.8 |
29.2±1.0 |
28.4±1.9 |
|
Lymphocytes, thousands |
1,804.8±75.9 |
1,532.7±52.2 |
1,696.5±47.2 (р=0.041)2 |
1,838.4±123.6 (р=0.004)2 |
|
CD3+, % |
72.5±2.0 |
67.7±1.3 (р=0.042) 1 |
70.4±0.8 |
68.6±2.0 |
|
CD3+, abs. |
1252.87±86.1 |
1033.2±45.8 (р<0.001)1 |
1,185.1±27.9 (р=0.003)2 |
1,331.2±112.9 (р=0.008)2 |
|
CD4+, % |
47.1±1.9 |
44.8±1.0 |
45.2±1.0 |
47.1±2.0 |
|
CD4+, abs. |
818.2±67.9 |
684.7±29.5 (р=0.007)1 |
750.9±16.2 (р=0.032)1 (р=0.041)2 |
911.8±78.9 (р=0.003)2 (р=0.004)3 |
|
CD8+, % |
22.9±1.9 |
20.1±0.7 (р=0.039)1 |
23.8±0.5 (р<0.001)2 |
20.4±0.6 (р<0.001)3 |
|
CD8+, absolute numbers |
391.2±38.6 |
304.5±16.7 (р<0.001)1 |
411.6±19.4 (р<0.001)2 |
396.7±38.3 |
|
CD4+/CD8+ |
2.24±0.23 |
2.38±0.10 |
1.97±0.07 (р<0.001)2 |
2.37±0.15 (р=0.036)3 |
|
CD19+, % |
11.7±1.04 |
11.1±0.8 |
10.5±0.4 |
9.5±1.1 |
|
CD19+, absolute numbers |
201.02±20.8 |
163.4±11.6 |
178.6±8.1 |
137.4±15.3 (р=0.041)3 |
|
CD16+56+, % |
13.9±1.6 |
19.7±1.4 (р=0.002)1 |
17.5±0.5 (р<0.001)2 |
17.8±1.4 (р=0.038)1 |
|
CD16+56+, absolute numbers |
244.3±40.4 |
313.9±35.1 |
307.3±17.6 |
299.8±8.8 |
|
Interleukin-4, pg/mL |
75.4±3.5 |
126.2±6.2 (р<0.001)1 |
120.5±5.1 (р<0.001)1 |
77.0±0.7 (р<0.001)2,3 |
|
Interleukin-6, pg/mL |
18.2±5.4 |
34.8±0.5 (р<0.001)1 |
62.4±2.2 (р<0.001)1 (р<0.001)2 |
72.1±4.4 (р<0.001)1 (р=0.005)3 |
|
Interleukin-10, pg/mL |
33.1±1.8 |
54.3±2.3 (р<0.001)1 |
55.8±1.7 (р<0.001)1 |
29.7±0.2 (р<0.001)1,2,3 |
|
Interleukin-17A, pg/mL |
211.3±40.9 |
378.6±8.4 (р<0.001)1 |
583.1±14.2 (р<0.001)1,2 |
584.5±19.2 (р<0.001)1,2,3 |
|
Tumor necrosis factor-α, pg/mL |
19.9±7.2 |
78.5±3.5 (р<0.001)1 |
79.6±2.6 (р<0.001)1 |
49.1±0.1 (р<0.001)1,2,3 |
|
Interferon-γ, pg/mL |
107.7±5.4 |
241.6±13.4 (р<0.001)1 |
225.3±11.8 (р<0.001)1 |
97.9±2.4 (р=0.036)1 (р<0.001)2,3 |
The changes of the cytokine profile in patients with mild to moderate grades of COPD were characterized by an increase in the content of IL-4, IL-6, IL-10, IL-17A, TNF-α, IFN-γ, compared with the control group. In the group with severe COPD, an excess of IL-6 and IL-17A was observed vs. mild COPD and moderate COPD groups. In patients with severe COPD, a reduction in the levels of IL-10 and IFN-γ was established, compared with the control group, with mild COPD group and moderate COPD group.
Fatty acid composition in leukocyte membranes of COPD patients
Among saturated SFAs, all patients with COPD, regardless of severity, showed an increase in the level of lauric (12:0) and stearic (18:0) acids, compared with the control group (Table 2). Moreover, the proportion of these FAs in the leukocyte cell membrane significantly increased in patients with moderate and severe COPD. The share of myristic acid (14:0) in patients with mild COPD was reduced vs. healthy patients, while in severe COPD, the percentages of 14:0 increased vs. the control group, mild COPDE group and severe COPD group. High level of palmitic acid (16:0) in the leukocyte membrane was observed in people with mild COPD. In the groups of patients with moderate and severe COPD, a reduction in the relative content of arachidic acid (20:0) was found, compared with the control group and the group of patients with mild COPD. Despite different vector of changes in the levels of individual SFAs, the total content of SFAs in all patients with COPD was enlarged vs. the control group.
Table 2. Fatty acid composition of leukocyte membrane and serum level of eicosanoids in patients with COPD
|
Indicators (% of total fatty acids) |
Control group n=32 |
Mild COPD n=50 |
Moderate CОPD n=63 |
Severe CОPD n=24 |
|
12:0 |
0.54±0.05 |
0.75±0.06 (р<0.001)1 |
0.9±0.01 (р<0.001)1 (р=0.009)2 |
0.8±0.01 (р<0.001)1 (р=0.008)3 |
|
14:0 |
1.91±0.21 |
1.5±0.12 (р=0.004)1 |
1.94±0.23 (р<0.001)1 |
2.31±0.32 (р<0.001)2 |
|
16:0 |
25.42±1.47 |
30.4±1 (р<0.001)1 |
25.51±1.59 (р<0.001)2 |
23.82±1.26 (р<0.001)2 (р=0.009)3 |
|
18:0 |
15.9±1.08 |
18.55±1.42 (р=0.005)1 |
20.45±1.09 (р<0.001)1 (р=0.009)2 |
23±2.18 (р<0.001)1 (р=0.007)2 (р=0.009)3 |
|
20:0 |
0.77±0.1 |
0.73±0.07 |
0.63±0.01 (р=0.007)1 (р=0.008)2 |
0.33±0.01 (р<0.001)1,2,3 |
|
16:1n-9 |
1.98±0.37 |
0.96±0.09 (р<0.001)1 |
1.21±0.14 (р<0.001)1 (р=0.007)2 |
1.3±0.18 (р<0.001)1,2 |
|
16:1n-7 |
1.48±0.09 |
0.78±0.08 (р<0.001)1 |
1.06±0.02 (р<0.001)1,2 |
1.37±0.03 (р<0.001)2 (р=0.003)3 |
|
18:1n-9 |
18.68±2.46 |
13.25±1.381 (р<0.001) |
11.78±0.88 (р<0.001)1 |
13.23±1.46 (р<0.001)1 |
|
18:1n-7 |
1.81±0.06 |
1.78±0.15 |
2.43±0.18 (р<0.001)1,2 |
2.35±0.13 (р<0.001)1,2,3 |
|
18:2n-6 |
12.96±2.44 |
8.97±0.96 (р<0.001)1 |
9.59±1.19 (р<0.001)1 |
9.02±0.84 (р<0.001)1 |
|
20:3n-6 |
0.41±0.03 |
1.34±0.02 (р<0.001)1 |
1.37±0.27 (р<0.001)1 |
0.96±0.01 (р<0.001)1,2,3 |
|
20:4n-6 |
5.52±0.33 |
13.87±1.57 (р<0.001)1 |
14.03±1.4 (р<0.001)1 |
17.07±0.96 (р<0.001)1 (р=0.003)2 (р=0.002)3 |
|
20:5n-3 |
0.86±0.03 |
0.47±0.05 (р<0.001)1 |
0.49±0.01 (р<0.001)1 |
0.35±0.01 (р<0.001)1 (р<0.001)2 (р=0.002)3 |
|
22:4n-6 |
0.41±0.02 |
1.46±0.15 (р<0.001)1 |
1.59±0.17 (р<0.001)1 |
1.41±0.2 р<0.001)1 |
|
22:5n-3 |
0.53±0.02 |
1.14±0.08 (р<0.001)1 |
1.07±0.03 (р<0.001)1 |
0.78±0.08 (р<0.001)1 (р<0.001)2 (р<0.001)3 |
|
22:6n-3 |
1.24±0.04 |
0.35±0.01 (р<0.001)1 |
0.51±0.01 (р<0.001)2 |
1.38±0.24 (р<0.001)2 (р<0.001)3 |
|
20:4n6/20:5n3 |
6.41±1.13 |
29.5±5.21 (р<0.001)1 |
28.6±5.21 (р<0.001)1 |
48.7±6.47 (р<0.001)1 (р<0.001)2 (р<0.001)3 |
|
Total SFAs |
44.54±2.34 |
51.93±1.91 (р<0.001)1 |
49.43±1.54 (р<0.001)1 |
50.26±2.14 (р<0.001)1 |
|
Total MUFAs |
23.95±1.98 |
16.77±0.54 (р<0.001)1 |
16.48±0.47 (р<0.001)1 |
18.25±0.74 (р<0.001)1 (р=0.006)2 (р=0.009)3 |
|
Total n-6 PUFAs |
19.3±2.4 |
25.64±3.5 (р=0.008)1 |
26.58±3.4 (р=0.007)1 |
28.46±4.1 (р=0.006)1 |
|
Total n-3 PUFAs |
2.63±0.14 |
1.96±0.15 (р=0.008)1 |
2.07±0.24 (р=0.006)1 |
2.51±0.2 (р=0.008)2 |
|
TXB2 |
15.5±1.2 |
32.1±2.4 |
27.9±2.9 |
34.2±3.1 |
|
LTB4 |
9.0±1.4 |
22.0±1.8 |
23.5±1.5 |
23.0±2.1 |
Values are expressed as % fatty acid of all FAs (mean ± SD).
1 p-value for the comparison vs. the control group; 2 p-value for the comparison vs. the mild COPD group; 3 p-value for the comparison vs. the moderate COPD group.
The level of monounsaturated fatty acids (MUFAs), such as hexadecenoic (16:1n-9) and oleic (18:1n-9) acids, were shown to decrease in all patients with COPD, regardless of the disease severity, as compared to the control group. The smaller percentage of palmitoleic acid (16:1n-7) in the membrane of immune cells was reported in patients with mild and moderate COPD. The groups with moderate and severe COPD demonstrated an increase of n-7 octadecenoic acid (18:1n-7) vs. the control group and mild COPD group. The total content of MUFAs in the leukocyte cell membrane was reduced in all COPD patients.
Modification of PUFA composition in the leukocyte membrane in COPD was characterized by statistically significant reduction in the levels of essential linoleic (18:2n-6), eicosapentaenoic (20:5n-3), and docosahexaenoic (22:6n-3) FAs. In the leukocyte membrane of COPD patients, accumulation of dihomo-γ-linolenic (20:3n-6), arachidonic (20:4n-6), docosatetraenoic (22:4n-6) and docosapentaenoic (22:5n-3) FAs was detected. Thus, content of individual n-3 PUFAs in the leukocyte membrane in patients with mild and moderate COPD declined significantly, which was also confirmed by the reduced total content of n-3 PUFAs. The total content of the studied n-6 PUFAs in all patients with COPD increased vs. the control group.
Changes in the ratio of 20:4n6/20:5n3 were revealed in all studied groups of patients with COPD. An increase in the ratio of 20:4n6 and 20:5n3 may indicate disorders in the synthesis of eicosanoids and the risk of inflammation. Arachidonic acid (20:4n6) is a precursor for the synthesis of proinflammatory and bronchoconstrictor eicosanoids (thromboxanes and leukotrienes), while eicosapentaenoic acid (20:5n-3) is a precursor for the synthesis of pro-resolving lipid mediators (maresins, resolvins and protectins). The balance shift between n-6 and n-3 PUFAs could be an unfavorable prognosis of an increased proinflammatory response.
To confirm the metabolic imbalance of FAs, we examined the content of TXВ2 and LTB4 in the blood serum of patients with COPD. We discovered that patients with COPD had higher concentrations of TXВ2 and LTB4 vs. the control group: patients with severe COPD had higher level of TXВ2 vs. patients with moderate COPD. Thus, the progression and exacerbation of COPD are accompanied by activation of the synthesis of proinflammatory and bronchoconstrictor lipid mediators.
Membrane potential of leukocyte mitochondria
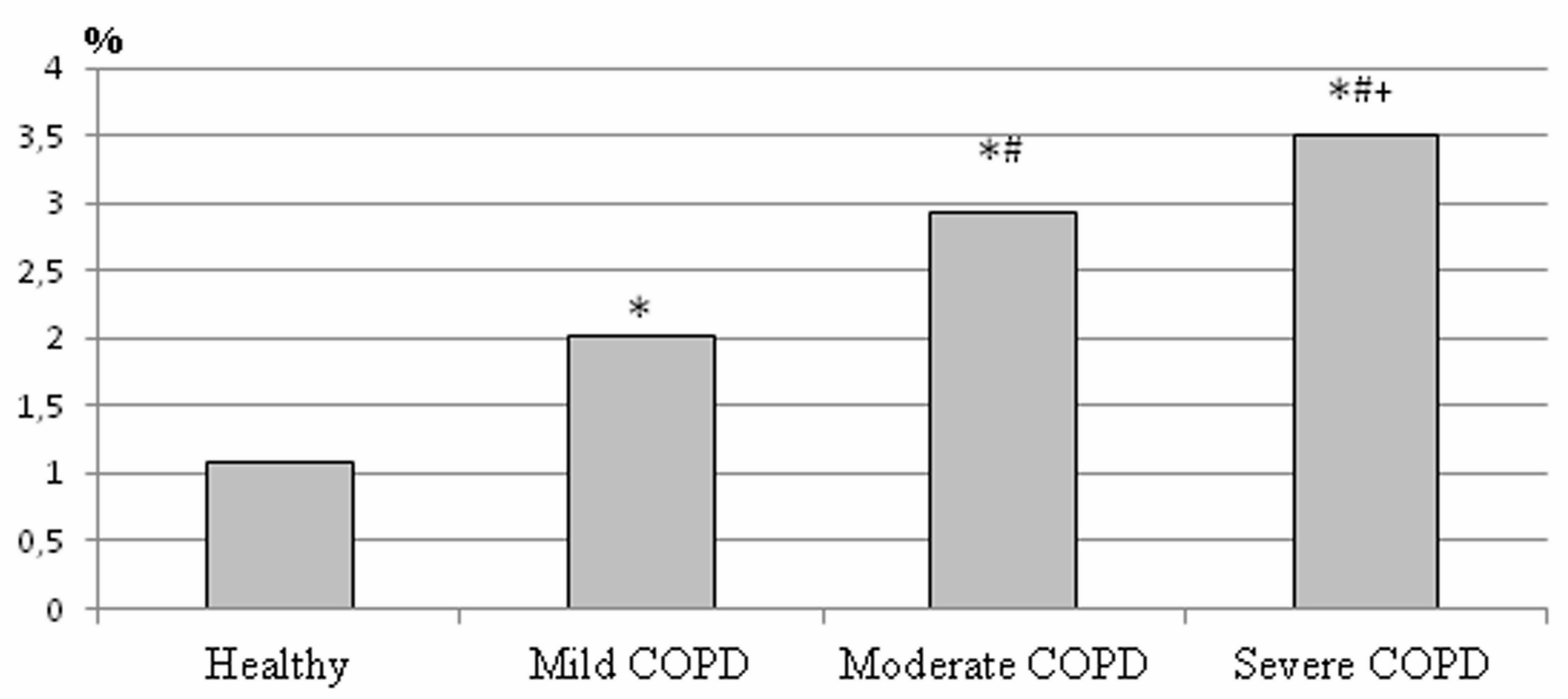
MMP is a marker of cell viability, optimal energy and redox processes, as well as a criterion of apoptosis [12, 15]. The determination of MMP is among the most suitable methods for studying the functional state of cells. It was found that the proportion of leukocytes with reduced MMP in patients with COPD increased with the progression of the disease (Figure 1). In patients with mild COPD, the proportion of cells with reduced MMP was 2.01%. In patients with COPD of moderate severity, MMP was 2.7 times higher vs. healthy people and amounted to 2.94%. In patients with severe COPD, 3.5% of leukocytes with reduced MMP were detected. An increase in the proportion of leukocytes with reduced MMP in patients with bronchopulmonary pathology implied impaired energy function of mitochondria, determining the mechanisms of cellular hypoxia and apoptosis of immune system cells [12].
Figure 1. The membrane potential of leukocyte mitochondria in patients with COPD.
*P<0.001 as compared to the control group; #P<0.001 as compared to the moderate COPD; +P<0.001 as compared to the mild COPD.
Correlation of fatty acids with immune status of COPD patients
In mild COPD, positive correlations were detected between the level of the lauric acid (12:0) and the content of IL-4 (r=0.6783; p=0.045) and IL-10 (r=0.7058; p=0.034); between myristic acid (14:0) and the overall leukocyte content (r=0.4229; p=0.05); between arachidic acid (20:0) and the lymphocyte numbers (r=0.4689; p=0.028); docosahexaenoic acid (22:6n-3) and the content of CD3+ (r=0.8001; p=0.003) and CD8+ (r=0.6921; p=0.018). Negative correlations were established between the content of palmitic acid (16:0) and the numbers of leukocytes (r=-0.5536; p=0.008), and CD4+ (r=-0.6401; p=0.034); between palmitoleic acid (16:1n-7) and the leukocyte numbers (r=-0.4737; p=0.026); between 16:1n-9 and the numbers of CD4+ (r=-0.6358; p=0.035); between 16:1n-9 and the CD19+ numbers (r=-0.6861; p=0.020). Docosapentaenoic acid (22:5n-3) manifested quite a few negative correlations with such cytokines as IL-4 (r= -0,6878, p=0,041), IL-10 (r= -0.7655, p=0.016), and IL-17А (r= -0.7864, p=0.012). The correlation analysis revealed that in mild COPD, the strongest relationship with immune parameters were exhibited by SFAs (12:0, 14:0, 16:0, 20:0), MUFAs (16:1n-7 и 16:1n-9), and n-3 PUFAs (22:5n-3 и 22:6n-3).
In moderate COPD, positive correlations were found between arachidic acid (20:0) and the numbers of lymphocytes expressed in % (r=0.5127; p=0.011) or in absolute numbers (r=0.4463; p=0.033); and the numbers of CD8+ (r=0.4523, p=0.030). Positive correlations were revealed between 18:1n-7 and the percentage of CD19+ (r=0.4905; p=0.017); between arachidonic acid (20:4n-6) and the percentage of CD3+ (r=0.6127, p=0.002) and CD4+ (r=0.5252, p=0,010); between docosatetraenoic acid (22:4n-6) and the percentage of lymphocytes (r=0.4890, p=0.018) and of CD4+ (r=0,5501, p=0,007). Weak negative correlations were demonstrated between the level of linoleic acids (18:2n-6) and content of IL-4 (r=-0.47, p=0.024) and IL-10 (r=-0.42, p=0.044), as well as between the level of arachidonic acids (20:4n-6) and IL-6 (r=-0.43, p=0.041), IL-10 (r=-0.48, p=0.020), and INF-γ (r=-0.44, p=0.037); also, between 18:1n-7 and the leukocyte content (r=-0.4883; p=0.018). The correlation analysis in patients with moderate COPD revealed the prevalence of correlations between n-6 PUFAs and the cytokine level, СD3+, СD4+, and СD8+, which was not the case in patients with mild COPD.
In severe COPD, positive correlation was observed between arachidonic acids (20:4n-6) and IL-4 (r=-0.4569, p=0.043); for myristic acid (14:0) with CD19+ level (r= 0.5182, p=0.048); for 16:1n-9 with lymphocyte content (r=0.5730; p=0.026); for linoleic acid (18:2n-6) with the lymphocyte content (r=0.5404; p=0.038), percentage of CD3+ (r=0.5710; p=0.026) and CD3+ in absolute numbers (r=0.5493; p=0.034), percentage of CD4+ (r=0.5600; p=0.030) and CD4+ in absolute numbers (r=0.5396; p=0.038), and CD8+ in absolute numbers (r=0.5717; p=0.026). Negative correlations were discovered between the level of myristic acid (14:0) and percentage of lymphocytes (r= -0.5222, p=0.046); between 16:1n-9 and percentage of CD8+ (r=-0.5268; p=0.044), percentage of CD19+ (r=-0.5774; p=0.024), and CD19+ in absolute numbers (r=-0.5597; p=0.030); between the linoleic acid (18:2n-6) content and IL-6 (r=-0.47, p=0.036). Thus, in patients with severe COPD, the relationship between immune parameters and essential linoleic acid prevailed, and a positive correlation appeared between arachidonic acid and IL-4, as well as between 14:0 and 16:1n-9 and cellular immunity.
Summing up the correlation indicators for assessing the immune system parameters and leukocyte membrane lipidome in patients with COPD, it can be concluded that as the course of the disease exacerbates, the values of the associations between n-6 PUFAs, cellular immunity, and some cytokines increase.
Discussion
Chronic obstructive pulmonary disease is a widespread pathology; it is often the cause of morbidity and mortality in the population, as well as the cause of early disability and significant medical costs [1, 2]. Obviously, only a comprehensive study of this disease could help finding a way out of this dead end.
The development of systemic inflammation is increasingly recognized as a key pathogenetic mechanism for the formation and progression of COPD [15]. However, the reason for the persistence of inflammation in COPD patients with long-term smoking cessation is still unknown. Weakness of the immune resolution is regarded a crucial factor in the development of various chronic diseases caused by systemic inflammation [2].
The inflammation in COPD is a complex of interrelated processes, involving responses of adaptive immunity and of plasma blood factors including cytokines [15]. We discovered a cytokine profile change in patients at different stages of COPD, which implied systemic inflammation. This finding was supported by the augmented level of proinflammatory cytokines: IL-6, IL-17А, TNF-α and IFN-γ vs. subjects in the healthy group.
Cytokines take part in the immune response regulation by transmitting a signal to the target cell via some specific receptor, providing differentiation of T helper (Th) cell subpopulations [16]. An increase in IL-4 and IL-10 from mild to moderate grade of COPD, induced largely by Th2 cells, suggested the development of an immune response of the Th2 type. Interleukin 4 (IL-4) and interleukin 10 (IL10) are principal in the formation of CD4+ type immunoreactivity. Despite the fact that these cytokines are considered crucial in the formation of the respiratory tract inflammation in bronchial asthma, they could possibly also affect the pathogenesis of the inflammatory response in COPD. The formation of СD4+ type immune response is very important for the development of the eosinophilic type of respiratory tract tissue inflammation, because it forms the COPD eosinophilic phenotype [15]. Besides, IL-4 activates production of growth factors contributing to airway remodeling [16].
An increase in the synthesis of IL-17A by Th17 cells in mild, moderate and severe COPD indicates the development of an immune response of the Th17 type. Typically, Th17 cells account for about 1% of CD3+CD4+ peripheral human cells and are characterized by predominant secretion of proinflammatory cytokines (TNFβ, IL-17A, IL-17F, IL-22, and others) [17]. Experimental and clinical studies demonstrated that IL-17 increases mucous expression of epithelial cells in the respiratory tract and activates protein production via the NF-κB-dependent pathway. Th17 cells activate airway eosinophilic inflammation caused by Th2 cells, and IL-17 intensify contraction and proliferation of airway smooth muscles, thereby making airway more permeable for allergens [18, 19]. Th17-type immune response deviation in exacerbating COPD suppresses differentiation of Th1 clones and organizes its own independent immune regulatory pathway via limiting the function of Th1 lymphocytes.
One of the reasons why leukocytes cannot adequately synthesize cytokines could be a change in the lipidome of the immune cell membrane [5]. A disbalance in the main processes of the FA cell metabolism influences greatly the processes of T cell differentiation and proliferation, and a further outcome of the immune response [6, 7]. Membrane lipids play sophisticated roles in regulating T cell signaling, such as development of the immunological synapse, recruitment of cytosolic signaling proteins, safety control of immunoreceptors, mediating protein island formation, as well as regulating the conformation, partitioning and mobility of membrane proteins. A single lipid molecule can have multiple regulatory functions.
Our findings demonstrated a considerable modification in the composition of SFAs, MUFAs, and PUFAs in the leukocyte membrane, occurring in patients with COPD. The revealed malfunction of leukocyte immune regulation could be due to a change in the FA status in their membrane. Lipids are known to serve as extracellular and intracellular messengers controlling the cell fate in both physiologically normal state and various pathologies [5, 6, 10]. Impaired lipid regulation of signal transmission leads to the development of oncological, cardiovascular, degenerative and bronchopulmonary pathologies, and inflammation [12].
The groups of patients with COPD demonstrated significant accumulation of SFAs. The predominance of SFAs in the leukocyte plasma membrane was responsible for a change in its permeability, viscosity, elasticity, expression, and receptor system functioning [12]. Similar disorders in the lipid matrix resulted in reduced cytomembrane stability and malfunction of ion channels, cell cooperation, and cell signaling. In vitro and in vivo data available in literature showed that an increase of SFA content in the macrophage membrane reduced their phagocytic ability, while an increased level of n-3 PUFAs in the cell membrane correlateв with an increased phagocytosis rate [5].
One way that SFAs affect chronic disease risk is through inflammation. A recent systematic review demonstrated a relationship between SFAs and C-reactive protein [5]. Healthy men on a diet with high contents of lauric, myristic and palmitic acids had increased concentrations of plasma C-reactive protein, fibrinogen and IL-6. Consumption of stearic acid alone, which does not seem to be hypercholesterolemic, surprisingly has a proinflammatory effect. To date, only a few studies linked SFA levels to systemic inflammation. Nevertheless, our study evidenced the SFA increase in the leukocyte membrane as an unfavorable symptom that becomes more apparent as the disease exacerbates.
Among MUFAs in the leukocyte membrane in patients with COPD, we revealed reduced levels of palmitoleic (16:1n-7), hexadecenoic (16:1n-9) and oleic (18:1n-9) acids. Most studies of the cell lipidome did not pay much attention to changing dynamics of MUFAs. However, the role of some MUFAs, such as oleic and palmitoleic ones, in maintaining cell integrity and functions has been confirmed [20]. For example, oleic acid plays critical metabolic and structural roles, being responsible for the reproductive function of the organism. When introduced into the diet, olive oil, rich in oleic acid, was shown to modulate a wide range of physiological functions, to reduce autoimmune and inflammatory responses, and to benefit the activity of immune cells. Antioxidant properties of oleic acid were confirmed in previous studies [20]. This acid is the best substratum for oxidation; therefore, it is one of the major acceptors of reactive oxygen species in vivo. In oxidative stress, the activation of ∆9-desaturase expression, along with increased synthesis of oleic acid, occur. Considerable reduction of this FA, revealed in our study, may imply insufficient antioxidant effect of leukocytes and exhausted potential against oxidative stress, which, in turn, negatively affects the cell architectonics and functional status.
Recent publications confirmed that palmitoleic acid (16:1n-7) plays an important role in metabolic and energy functions of the cell. It increases the cell energy activity via a coordinated effect on lipolysis, FA etherification, and their oxidation in mitochondria [5]. Our findings demonstrated the 16:1n-7 deficiency in the leukocyte membrane, which may have indirectly indicated an impaired energy function of leukocytes.
The direct evidence of impaired energy processes in immune cell mitochondria, revealed in our study, was an increase of leukocyte quantity with reduced MMP.
We detected a considerable impairment in the composition of PUFAs in the leukocyte membrane of patients with COPD. Modification of their composition is characterized by lower levels of linoleic (18:2n-6), eicosapentaenoic (20:5n-3) and docosahexaenoic (22:6n-3) acids, along with higher levels of most n-6 PUFAs (20:3n-6, 20:4n-6, 22:4n-6). Cell membrane PUFAs are known to influence fundamental properties of the plasma membrane, including membrane fluidity, protein functionality, and lipid raft signaling [6, 7]. Dietary n-3 PUFAs can target the plasma membrane of immune cells by altering plasma membrane lipid dynamics, thereby regulating the reduction of immune cell activation and suppressing the inflammation [10]. We revealed a change in the FA composition mostly towards the reduced number of essential PUFAs esterified into phospholipids, which cannot but lead to the impaired synthesis of proinflammatory and pro-resolving lipid mediators. Activation of metabolic changes in n-6 PUFAs under bronchopulmonary pathology is confirmed by the simultaneous depletion of the 18:2n-6 level and the increased synthesis of 20:4n-6 and 22:4n-6. A higher content of 20:4n-6 and its metabolite, 22:4n-6, in the leukocyte membrane in patients with COPD implied increasing levels of the precursor for the formation of inflammatory mediators, such as TXB2 and LTB4, which was confirmed by their higher levels in the blood of patients with COPD.
The increased content of arachidonic acid (20:4n6) in the leukocyte membrane occurred in conjunction with substantial deficiency of its main competitor, eicosapentaenoic acid (20:5n3). The shortage of eicosapentaenoic acid as the main source for the synthesis of pro-resolving lipid mediators (resolvins, maresins and protectins) in the immune cell membrane determined impaired resolution of the inflammation, which triggered the development of chronic inflammatory process.
The correlation revealed between the examined parameters of the immune system and the status of leukocyte FAs indicated high importance of FAs and their derivatives in systemic inflammation development in COPD. The relationships between n-6 PUFAs, cell immunity and cytokines were shown to form with the disease progression. As COPD aggravated, arachidonic acid and its derivatives played a leading role in regulating the immune process, thereby shifting cellular immune signaling towards the activation of proinflammatory responses. Without correlation between n-3 PUFAs and immunity in moderate and severe COPD, a disorder of the inflammation resolution processes occurred. This disorder implied the imbalance between n-6 PUFAs and n-3 PUFAs, and the synthesis of proinflammatory and pro-resolving oxylipins. The dynamic balance was shifting towards accumulation of the precursor of proinflammatory eicosanoids, initiating pathogenic mechanisms of the development and progression of immune impairment in COPD, thereby becoming one of the key factors in forming systemic inflammation.
Conclusion
The results of our study showed that the FA composition disorder of the leukocyte membrane begins to manifest itself in patients with mild COPD. The breakdown in FA metabolism and plasma membrane architectonics, identified in mild COPD patients, aggravated as the disease progressed, and were directly related to the development of systemic inflammation. We established that the mechanism of development and progression of COPD was mediated by an imbalance in the FA composition of leukocytes and impaired eicosanoid synthesis. Modification of FA composition in patients with chronic pathology of the bronchopulmonary system led not only to a disruption in the structure of the cellular skeleton, but also to the pathology of the immune response regulation. Disorganization of the lipid component in the cell membrane caused the development of the inflammatory process, which was the key factor in the pathogenesis of COPD.
Ethical approval
All procedures in clinical studies were in accordance with the ethical standards of the institutional and/or national research committee and with 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Study limitations
Small sample size is a main limitation of our study.
Funding
The study did not have external funding.
Conflict of interest
The authors declare that they have no conflicts of interest.
- Global Initiative for chronic obstructive lung disease. Pocket guide to COPD diagnosis, management, and prevention. A guide for health care professionals. 2020. https://goldcopd.org/gold-reports/.
- Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol 2016; 138(1): 16-27. https://doi.org/10.1016/j.jaci.2016.05.011.
- Kytikova O, Novgorodtseva T, Denisenko Y, Antonyuk M, Gvozdenko T. Pro-resolving lipid mediators in the pathophysiology of asthma. Medicina (Kaunas) 2019; 55(6): 284. https://doi.org/10.3390/medicina55060284.
- Nambiar S, Bong How S, Gummer J, Trengove R, Moodley Y. Metabolomics in chronic lung diseases. Respirology 2020; 25(2): 139-148. https://doi.org/10.1111/resp.13530.
- Rosa Neto JC, Calder PC, Curi R, Newsholme P, Sethi JK, Silveira LS. The Immunometabolic roles of various fatty acids in macrophages and lymphocytes. Int J Mol Sci 2021; 22(16): 8460. https://doi.org/10.3390/ijms22168460.
- Schumann J. It is all about fluidity: Fatty acids and macrophage phagocytosis. Eur J Pharmacol 2016; 785: 18-23. https://doi.org/10.1016/j.ejphar.2015.04.057.
- Hou TY, McMurray DN, Chapkin RS. Omega-3 fatty acids, lipid rafts, and T cell signaling. Eur J Pharmacol 2016; 785: 2-9. https://doi.org/10.1016/j.ejphar.2015.03.091.
- Kytikova OY, Perelman JM, Novgorodtseva TP, Denisenko YK, Kolosov VP, Antonyuk MV, Gvozdenko TA. Peroxisome proliferator-activated receptors as a therapeutic target in asthma. PPAR Res 2020; 2020: 8906968. https://doi.org/10.1155/2020/8906968.
- Fan YY, Fuentes NR, Hou TY, Barhoumi R, Li XC, Deutz NEP, et al. Remodelling of primary human CD4+ T cell plasma membrane order by n-3 PUFA. Br J Nutr 2018; 119(2): 163-175. https://doi.org/10.1017/s0007114517003385.
- Wood LG. Omega-3 polyunsaturated fatty acids and chronic obstructive pulmonary disease. Curr Opin Clin Nutr Metab Care 2015; 18(2): 128-132. https://doi.org/10.1097/mco.0000000000000142.
- Gangopadhyay S, Vijayan VK, Bansal SK. Lipids of erythrocyte membranes of COPD patients: A quantitative and qualitative study. COPD 2012; 9(4): 322-331. https://doi.org/10.3109/15412555.2012.668581.
- Novgorodtseva TP, Denisenko YK, Zhukova NV, Antonyuk MV, Knyshova VV, Gvozdenko TA. Modification of the fatty acid composition of the erythrocyte membrane in patients with chronic respiratory diseases. Lipids Health Dis 2013; 12: 117. https://doi.org/10.1186/1476-511x-12-117.
- Van der Does AM, Heijink M, Mayboroda OA, Persson LJ, Aanerud M, Bakke P, et al. Dynamic differences in dietary polyunsaturated fatty acid metabolism in sputum of COPD patients and controls. Biochim Biophys Acta Mol Cell Biol Lipids 2019; 1864(3): 224-233. https://doi.org/10.1016/j.bbalip.2018.11.012.
- Wu W, Shi X, Xu C. Regulation of T cell signalling by membrane lipids. Nat Rev Immunol 2016; 16(11): 690-701. Erratum in: Nat Rev Immunol. 2018. https://doi.org/10.1038/nri.2016.103.
- Agarwal AR, Kadam S, Brahme A, Agrawal M, Apte K, Narke G, et al. Systemic Immuno-metabolic alterations in chronic obstructive pulmonary disease (COPD). Respir Res 2019; 20(1): 171. https://doi.org/10.1186/s12931-019-1139-2.
- Ul-Haq Z, Naz S, Mesaik MA. Interleukin-4 receptor signaling and its binding mechanism: A therapeutic insight from inhibitors tool box. Cytokine Growth Factor Rev 2016; 32: 3-15. https://doi.org/10.1016/j.cytogfr.2016.04.002.
- Tan HL, Rosenthal M. IL-17 in lung disease: Friend or foe? Thorax 2013; 68(8): 788-790. https://doi.org/10.1136/thoraxjnl-2013-203307.
- Sidletskaya KA, Vitkina TI, Denisenko YK, Mineeva EE. Role of toll-like receptor 2 in regulation of T-helper Immune response in chronic obstructive pulmonary disease. Can Respir J 2021; 2021: 5596095. https://doi.org/10.1155/2021/5596095.
- Sidletskaya K, Vitkina T, Denisenko Y. The Role of toll-like receptors 2 and 4 in the pathogenesis of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2020; 15: 1481-1493. https://doi.org/10.2147/copd.s249131.
- Sales-Campos H, Souza PR, Peghini BC, da Silva JS, Cardoso CR. An overview of the modulatory effects of oleic acid in health and disease. Mini Rev Med Chem 2013; 13(2): 201-210. https://pubmed.ncbi.nlm.nih.gov/23278117.
Received 25 March 2022, Revised 31 May 2022, Accepted 10 October 2022
© 2022, Russian Open Medical Journal
Correspondence to Nataliya V. Bocharova. E-mail: natellav@inbox.ru.